Vascular Intervention // Coronary
Semi-Compliant Workhorse Balloon Catheter
Pantera Pro

 Better crossability in tight lesions
Better crossability in tight lesions 43% less friction during kissing balloon technique
43% less friction during kissing balloon technique 38% more push to reach target lesion
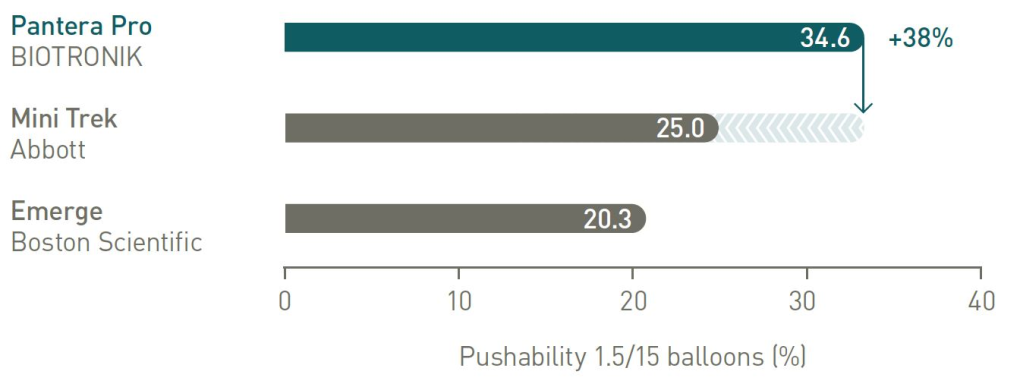
38% more push to reach target lesion
Better crossability in tight lesions1
Slim shoulders and hydrophilic coating
Proprietary balloon material for small sizes allows for slim shoulders while maintaining durability. Coupled with hydrophilic balloon coating, Pantera Pro excels in tight lesions.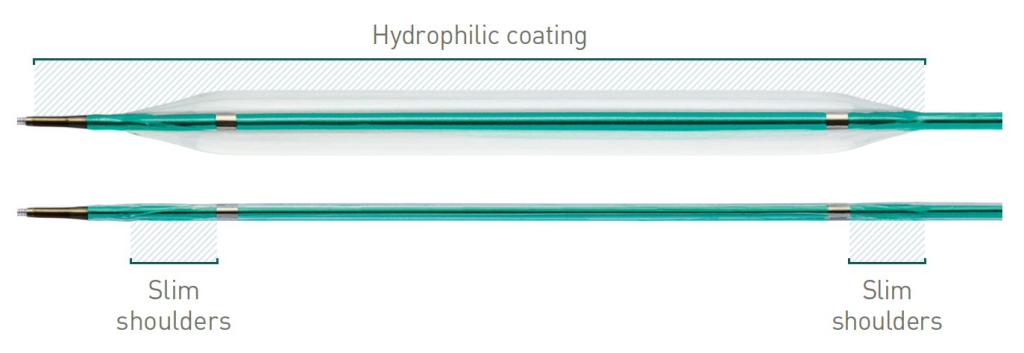

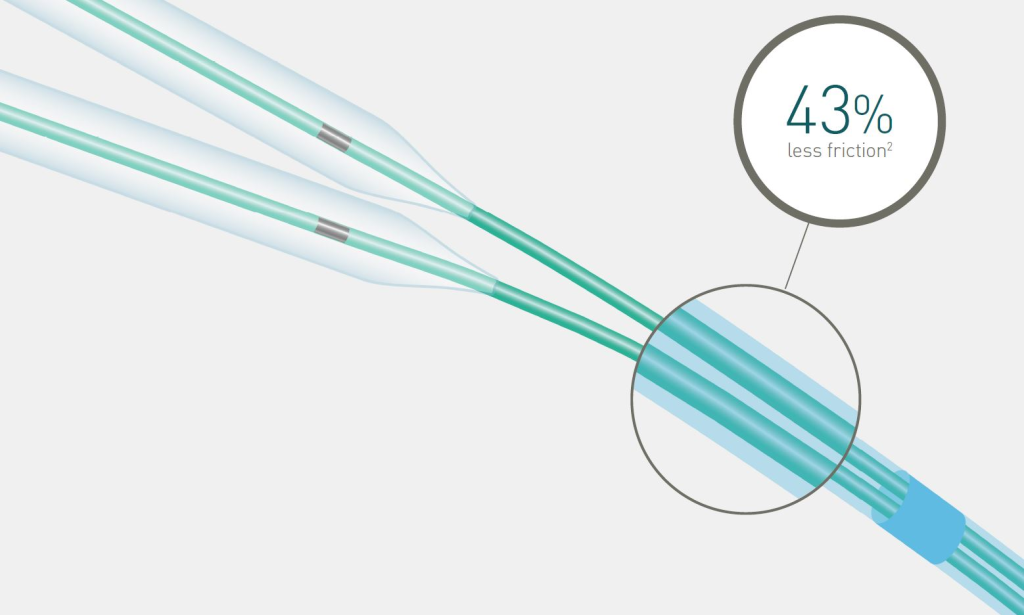
43% less friction2 during kissing balloon technique
Reduced distal shaft profile
The reduced distal shaft profile lowers friction when using two balloons in a 6F guiding catheter.*
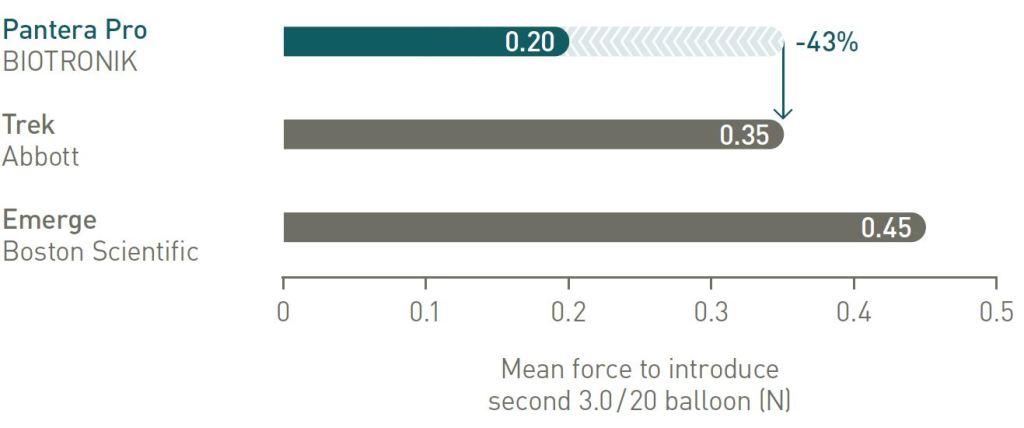
Lowest friction during kissing balloon technique compared to main competitors
*Any combination of two diameters not larger than 3.5 mm within a 6F guiding catheter with a minimal inner diameter of 0.070”/1.78 mm.
38% more push3 to reach target lesion
Enhanced Force Transmission shaft
BIOTRONIK’s unique Enhanced Force Transmission shaft results in optimal pushability due to the direct transition from proximal metallic hypotube to distal guide wire support section.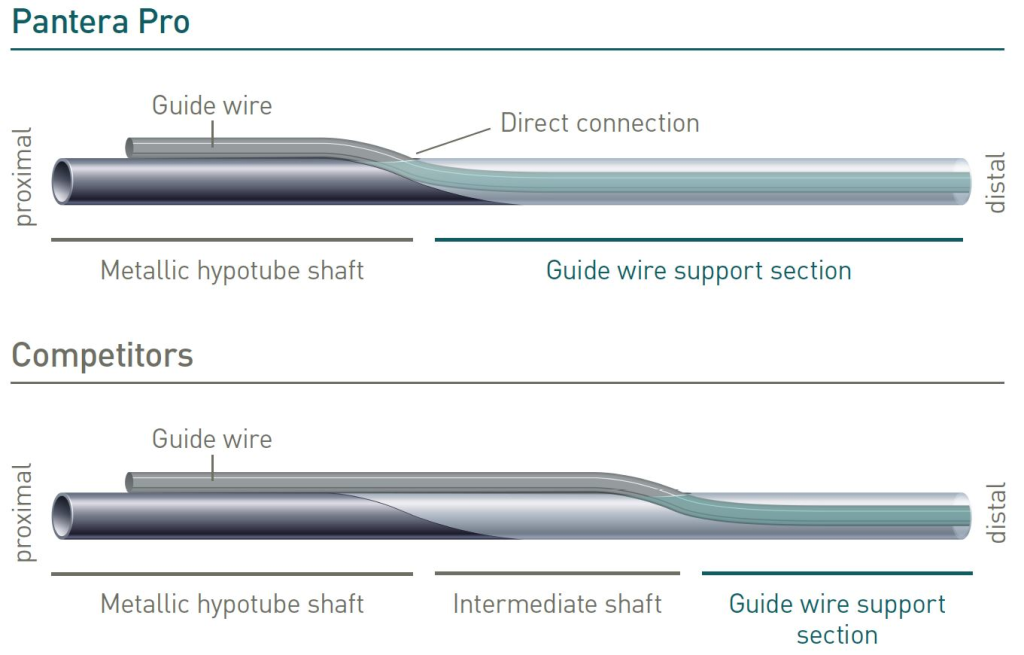
Pushability comparison

 Pantera Pro
Pantera ProIndicated for dilation of coronary artery or bypass graft stenosis.*
Technical Data
| Proximal Shaft | |
|---|---|
| Design | Hypotube design |
| Diameter | 2.0F |
| Shaft markers | 92 cm and 102 cm from tip |
| Distal Shaft | |
|---|---|
| Guiding catheter | 5F (min. I.D. 0.056”/1.42 mm) |
| Guide wire diameter | 0.014” |
| Lesion entry profile | 0.017” |
| Usable length | 140 cm |
| Balloon material | Semi Crystalline Co-Polymer |
| Balloon folding | ø 1.25 - 1.5 mm: Two-fold; ø 2.0 - 4.0 mm: Tri-fold |
| Balloon markers | Platinum-Iridium: ø 1.25 - 1.5 mm one marker; ø 2.0 - 4.0 mm two markers |
| Coating distal shaft | Hydrophilic (end of balloon to Guide Wire (GW) exit port) |
| Balloon and tip coating | ø 1.25 - 2.0 mm: Hydrophilic ø 2.50 - 4.0 mm: Hydrophobic |
| Kissing balloon technique | 6F guiding catheter (min. I.D. 0.070”/1.78 mm), up to ø 3.5 mm |
| Diameter | 2.6F (ø 1.25 - 2.0 mm); 2.7F (ø 2.5 - 3.5 mm); 2.9F (ø 4.0 mm) |
Compliance Chart
Ordering Information
| 1.25 | 393289 | 393291 | 393298 | 393305 | – | – | ||||||||
| 1.5 | 393290 | 393292 | 393299 | 393306 | – | – | ||||||||
| 2.0 | – | 393293 | 393300 | 393307 | 393312 | 393317 | ||||||||
| 2.5 | – | 393294 | 393301 | 393308 | 393313 | 393318 | ||||||||
| 3.0 | – | 393295 | 393302 | 393309 | 393314 | 393319 | ||||||||
| 3.5 | – | 393296 | 393303 | 393310 | 393315 | 393320 | ||||||||
| 4.0 | – | 393297 | 393304 | 393311 | 393316 | 393321 | ||||||||
Contact
1 1.25-2.0 mm diameter, bench test when compared to key competitors, BIOTRONIK data on file;
2 vs Trek (Abbott), BIOTRONIK data on file; 3. vs Mini Trek (Abbott), BIOTRONIK data on file.
Trek and Mini Trek are registered trademarks of Abbott; Emerge is a registered trademark of Boston Scientific.
*Indication as per IFU.

