Product Details
Easy Release
Relieves friction of introducer valve on the retractable shaft during stent deployment, providing a smoother stent release.

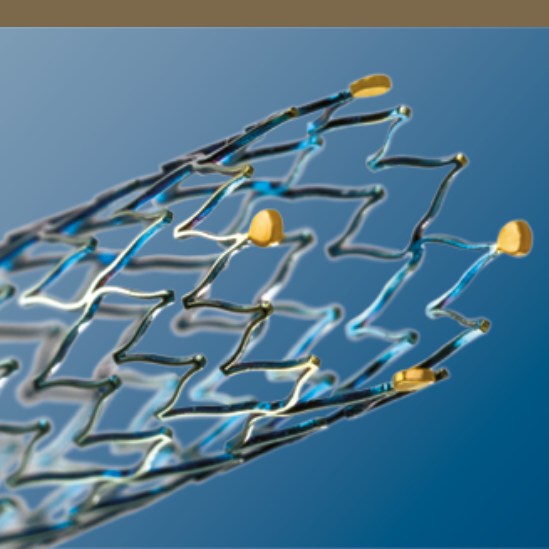
Segmented Stent Design for Iliac Artery
- Peak-to-valley design and S-articulating connecting bars provide multi-directional flexibility and avoid fish-scaling in tortuous arteries.
- Segmented design and strut thickness designed to provide sufficient chronic outward force.

Stent Visibility
Four gold markers at each end of the stent enhance visibility.
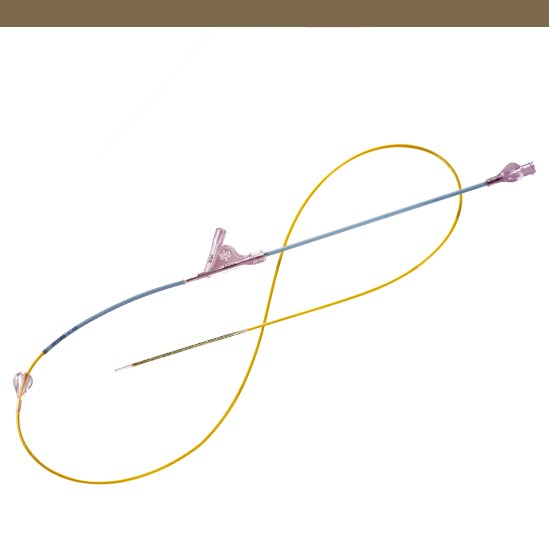
Pull-Back Delivery System
Enables simple stent deployement.
Safety Tab
Designed to prevent accidental stent deployment.

proBIO reduces ion release
- proBIO reduces release of Nickel and other metal ions from the stent.
- proBIO acts as an effective and reliable barrier to Nickel and other heavy metal ion diffusion.
6 F Introducer Compatibility
6F distal shaft with 5.2F proximal shaft allows contrast injection while the device is positioned inside the introducer and across the lesion.
BIOFLEX-I (US-IDE)
- Investigational Device Exemption Study to Determine the Safety and Efficacy of the Astron and Pulsar Stents.
- The objective of this study is to separately demonstrate the safety and efficacy of BIOTRONIK Astron and Pulsar stents.
- The Pulsar stent will be used for the treatment of femoro-popliteal lesions, located in the native superficial femoral artery (SFA) or proximal popliteal artery (PPA), while the Astron stent will be used for the treatment of the common or external iliac artery lesions.
- Estimated enrollment: 456
BIOFLEX-I EU
- A Study to Determine the Performance of the Astron and Pulsar-18 Stents in Europe.
- BIOFLEX-I EU is the European arm of the BIOFLEX-I IDE study (NCT01319812).
- Data from BIOFLEX-I EU will be pooled with data in the IDE.
- The objective of this study is to separately demonstrate the clinical performance of BIOTRONIK's Astron and Pulsar-18 stents in the European arm of the BIOFLEX-I IDE (NCT01319812).
- The Pulsar-18 stent will be used for the treatment of femoro-popliteal lesions, located in the native superficial femoral artery (SFA) or proximal popliteal artery (PPA), while the Astron stent will be used for the treatment of the common or external iliac artery lesions. Estimated enrollment: 456
4-EVER
- A Trial Investigating the Safety of 4 F Endovascular Treatment of Infra-Inguinal Arterial Stenotic Disease.
- The objective of this clinical investigation is to evaluate the puncture site complication rate as well as the short- and long-term (up to 24 months) treatment outcomes by means of Astron Pulsar/Pulsar-18 stent implantation in symptomatic (Rutherford 2-4) femoro-popliteal arterial stenotic or occlusive lesions, using BIOTRONIK 4 F compatible devices and without the use of a closure device.
- The hypothesis is that the primary patency at 12 months is non-inferior to the primary patency obtained in the Durability study (72.2%).
- Enrollment: 120
Technical Data
| Astron Stent | |
|---|---|
| Catheter type |
OTW |
| Recommended guide wire |
0.035" |
| Stent material |
Nitinol |
| Strut thickness |
225 μm (ø 10 mm = 230 μm) |
| Stent coating |
proBIO (amorphous silicone carbide) |
| Stent markers |
4 gold markers each end |
| Sizes |
ø 7 - 10 mm; L: 30 - 80 mm |
| Proximal shaft |
5.2 F, hydrophobic coating |
| Usable length |
70 and 120 cm |
Order Information
Contact
1 Nickel release test results, BIOTRONIK data on file.