Key Results
Key Result 1
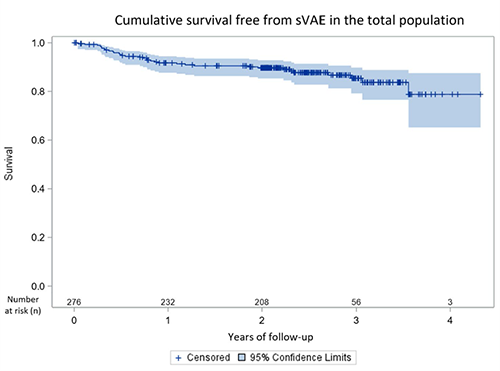
The rate of patients with sustained ventricular arrhythmia events (sVAE) was 8.3%, 10.3%, and 21.2% at 1, 2, and 4 years post replacement.
BIOtronik Study to Assess the CONTINUation of Existing Risk of Ventricular Arrhythmias After CRT-D Replacement for Patients With Primary Prevention Indication
Gras, D., Clémenty, N., Ploux, S. et al. CRT-D replacement strategy: results of the BioCONTINUE study. J Interv Card Electrophysiol (2022)
The rate of patients with sustained ventricular arrhythmia events (sVAE) was 8.3%, 10.3%, and 21.2% at 1, 2, and 4 years post replacement.

Patients without persistent ICD indication at replacement still had a sVAE rate of 5.7% (95% CI 2.3–11.5%) at 2 years.
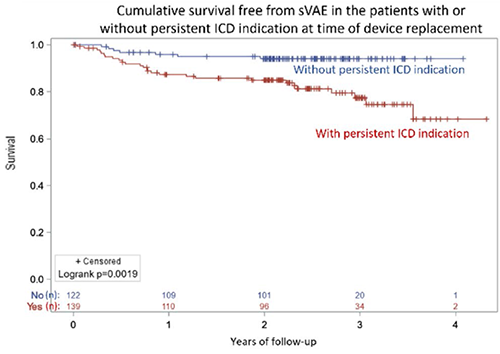
In multivariate analysis, predictive factors of subsequent sVAE were:
Rate of patients with at least one sustained ventricular arrhythmic event (sVAE) in patients with a