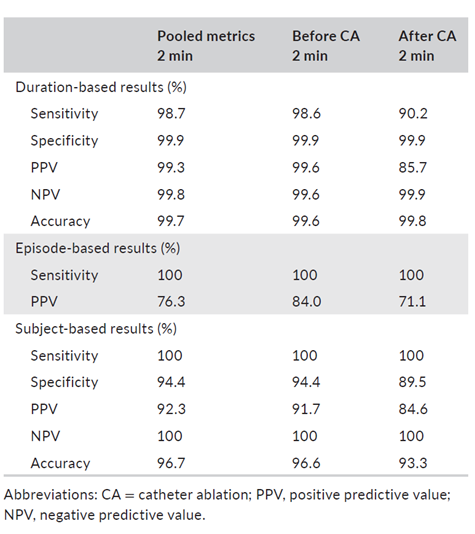
Key Results
Key Result 1
All true AF episodes were detected by the BIOMONITOR III
- 100% episode-based sensitivity
The BIOMONITOR III identified all patients with AF
- 100% patient-based sensitivity
BIOMONITOR III provided highly accurate AF burden estimation before and after catheter ablation
- 99.6% duration-based accuracy before catheter ablation
- 99.8% duration-based accuracy after catheter ablation