Magmaris®
Magmaris ist das erste klinisch bewährte bioabsorbierbare Magnesium-Scaffold.1-4 Es ist derzeit für De-Novo-Läsionen mit einem Referenzgefäßdurchmesser und einer Läsionslänge zugelassen, die genau den verfügbaren Magmaris-Größen entsprechen.ᵃ⁵
Produkt-Highlights
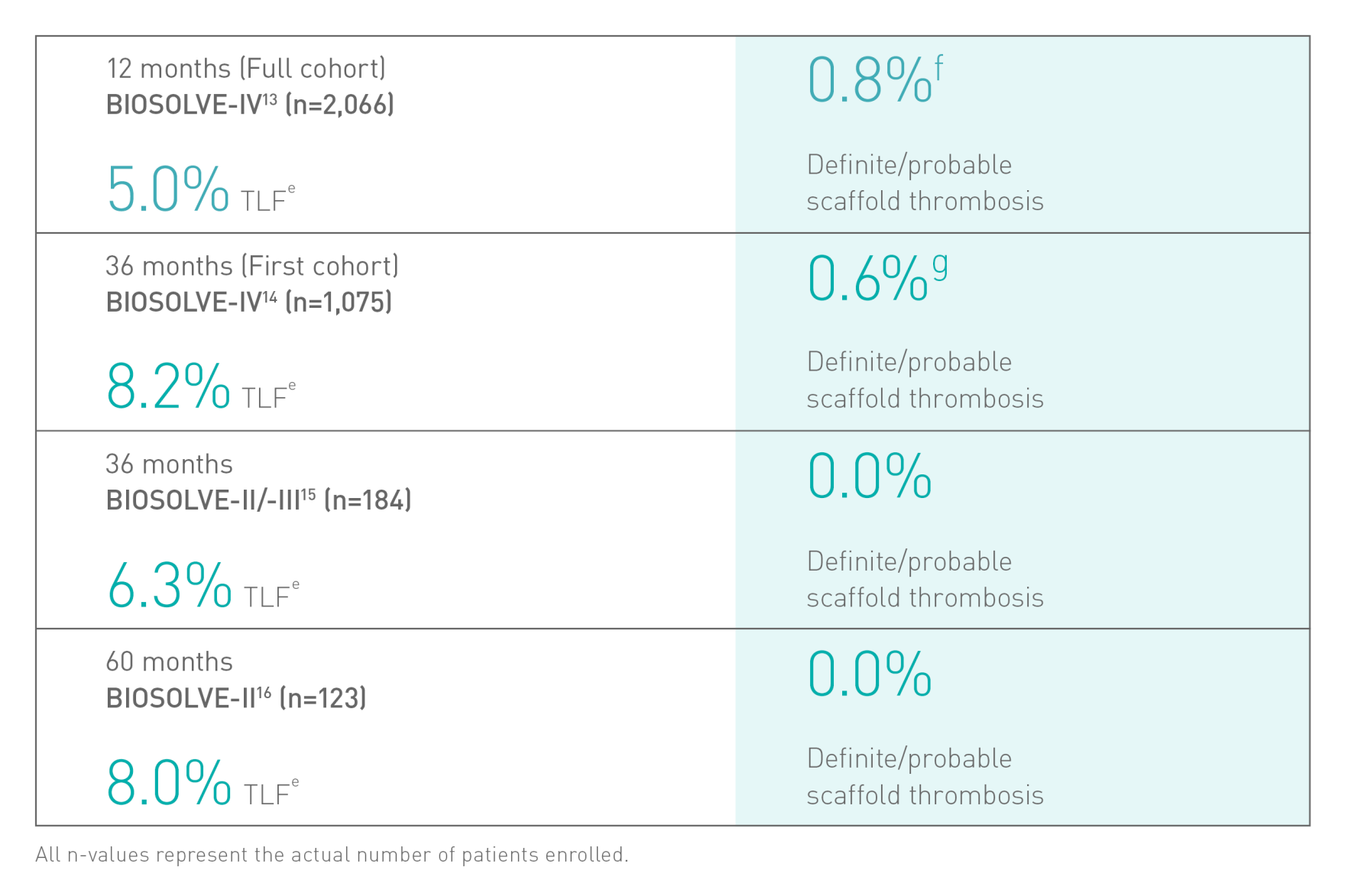
Sicherheit und Wirksamkeit klinisch belegtb6-8
Nachweisliche Sicherheit
Kurze Magnesiumresorptionszeit
~95 % des Magnesiums sind nach 12 Monaten resorbiert9
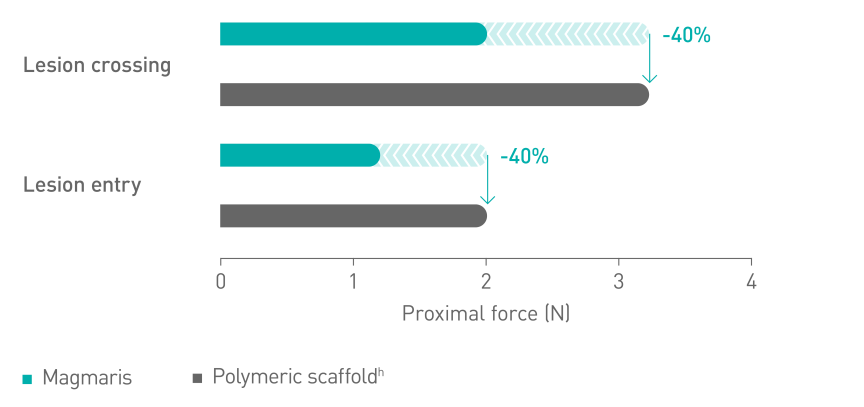
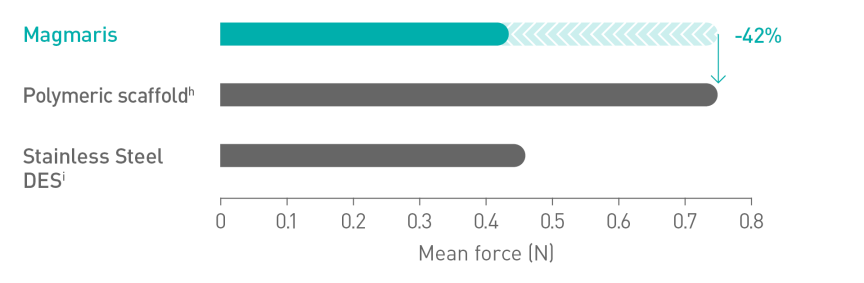
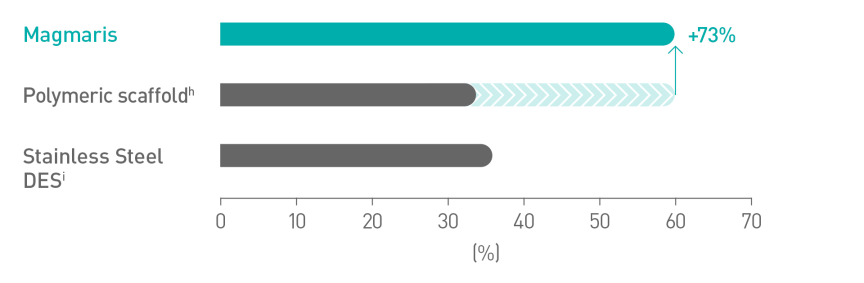
Bessere Platzierbarkeitc10
Magnesium ermöglicht eine glattere Scaffold-Oberfläche11
Produktüberblick
Magmaris

Röntgendichte Doppel-Marker
Magnesiumgerüst
BIOlute®-Beschichtung
Technische Daten
| Scaffold | |
|---|---|
| Scaffoldmaterial | Unternehmenseigene Magnesiumlegierung |
| Marker | Zwei Tantalmarker an jedem Ende |
| Aktive Beschichtung | BIOlute® (resorbierbares Poly-L-Lactid (PLLA), das einen Limus-Wirkstoff freisetzt) |
| Wirkstoffkonzentration | 1,4 μg/mm2 |
| Strebendicke/-breite | 150 μm / 150 μm |
| Durchmesser bei maximaler Aufdehnung |
Nenndurchmesser +0,6 mm |
| Einführsystem | |
| Kathetertyp | Rapid-Exchange |
| Empfohlener Führungskatheter | 6 F (min. ID: 0,070") |
| Crossing-Profil | 1,5 mm |
| Führungsdraht-Durchmesser | 0,014” |
| Katheter-Arbeitslänge | 140 cm |
| Ballonmaterial | Semikristallines Co-Polymer |
| Beschichtung (distaler Schaft) | Doppelt beschichtet |
| Markierungsbänder | Zwei eingebettete Platin-Iridium-Marker |
| Proximaler Schaftdurchmesser | 2,0 F |
| Distaler Schaftdurchmesser | 2,9 F |
| Nominaldruck (NP) | 10 atm |
| Nenn-Berstdruck (RBP) | 16 atm |
Compliance-Tabelle
| Balloon diameter (mm) | |||
|---|---|---|---|
| ø 3,00 | ø 3,50 | ||
| Nominaldruck (NP) | atm* | 10 | 10 |
| ø (mm) | 3,00 | 3,54 | |
| Nenn-Berstdruck (RBP) | atm* | 16 | 16 |
| ø (mm) | 3,29 | 3,82 | |
| *1 atm = 1,013 bar | |||
Bestellinformationen
| Scaffold- ø (mm) |
Scaffold- länge (mm) |
||
|---|---|---|---|
| 15 | 20 | 25 | |
| 3,00 | 412526 | 412527 | 412528 |
| 3,50 | 412529 | 412530 | 412531 |
Downloads und weitere Links
Downloads
Weitere Links
Medien
Produktanimationen
Wie können wir Ihnen helfen?
Referenzen
a. Indikation gemäß Gebrauchsanweisung. b. Auf Basis von BIOSOLVE-II, -II/-III und -IV, zu Patientenpopulationen siehe Angaben zur Studie. c. Im Vergleich zu einem führenden Polymer-Scaffold - Abbott Absorb. d. 2-Jahres-Follow-up der Gesamtkohorte der BIOSOLVE-IV-Studie (n=2.066 Patienten). e. Zielläsionsversagen (TLF) definiert als Zusammensetzung aus Herztod, Zielgefäß-Myokardinfarkt (TV-MI), Notfall-Bypass (eCABG) und klinisch bedingter Revaskularisierung der Zielläsion (CD-TLR). f. 0,4 % Prozent der Fälle ohne frühe Aggregationshemmer- oder Antikoagulationsunterbrechung nach dem Eingriff. g. 0,5 % Scaffoldthromboserate ohne Fälle mit früher Aggregationshemmer- oder Antikoagulationsunterbrechung. h. Absorb, Abbott; i. BioFreedom, Biosensors.
1. Erbel R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2. Juni 2007;369(9576):1869-1875. doi: 10.1016/S0140-6736(07)60853-8. 2. Haude M et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first in-man BIOSOLVE-I trial. Lancet. 9. März 2013;381(9869):836-44. 3. Wang et al. Vascular restoration therapy and bioresorbable vascular scaffold. Regenerative Biomaterials, 2014, 49–55. doi: 10.1093/rb/rbu005. 4. Haude M et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month|results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet.2016;Jan 2;387(10013):31-9. doi: 10.1016/S0140-6736(15)00447-X. Epub 12. Oktober 2015. 5. Fajadet J et al._Magmaris preliminary recommendation upon commercial launch: a consensus from the expert panel on 14 April 2016. EuroIntervention. 2016;12:828-833. 6. Torzewski J. Safety and performance of Magmaris at 24 month follow up of BIOSOLVE IV. Präsentiert auf eEuroPCR 2021, virtueller Kongress. ClinicalTrials.gov: NCT02817802. 7. Haude M. Long-term clinical data of the BIOSOLVE-II study with the drug-eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries - BIOSOLVE-II. Präsentiert auf: e-Course PCR, 25. Juni 2020, Paris, Frankreich. 8. Haude M, et al. Sustained safety and performance of the second-generation sirolimus-eluting absorbable metal scaffold: Pooled outcomes of the BIOSOLVE-II and -III trials at 3 years. Cardiovascular Revascularization Medicine. 2020. doi:10.1016/j.carrev.2020.04.006. 9. Joner M, Ruppelt P, Zumstein P, et al. Preclinical Evaluation of Degradation Kinetics and Elemental Mapping of First and Second Generation Bioresorbable Magnesium Scaffolds. EuroIntervention. 20. Februar 2018. pii: EIJ-D-17-00708. doi: 10.4244/EIJ-D-17-00708. [Elektronische Veröffentlichung vor dem Druck]. 10. Schmidt et al. In vitro performance investigation of bioresorbable scaffolds – standard tests for vascular stents and beyond. Cardiovasc Revasc Med. 2016 Sep;17(6):375-83. doi: 10.1016/j.carrev.2016.05.001. Epub 13. Mai 2016. 11. BIOTRONIK Daten im Archiv. 12. Bennett J. Safety and Efficacy of the Resorbable Magnesium Scaffold, Magmaris in a Real-World Setting – 24-month Follow-up of the Full Cohort (2066 subjects) of the BIOSOLVE-IV Registry. Präsentiert auf: TCT, September 2022, Boston, USA. ClinicalTrials.gov: NCT02817802. 13. Bennett J. Performance and safety of the resorbable magnesium scaffold, Magmaris in a real-world setting – Primary and secondary endpoint analysis of the full cohort (2,066 subjects) of the BIOSOLVE-IV. Präsentiert auf: TCT 2021, November 2021, Orlando, USA. ClinicalTrials.gov: NCT02817802. 14. Torzewski J. Safety and performance of Magmaris at 36-months: BIOSOLVE-IV first cohort. Präsentiert auf: EuroPCR; 2022; ClinicalTrials.gov: NCT02817802. 15. Haude M, et al. Sustained safety and performance of the second-generation sirolimus-eluting absorbable metal scaffold: Pooled outcomes of the BIOSOLVE-II and -III trials at 3 years. Cardiovascular Revascularization Medicine. 2020. doi: 10.1016/j.carrev.2020.04.006. 16. Haude M, et al. Sustained safety and performance of a second-generation sirolimus-eluting absorbable metal scaffold: Long-term data of the BIOSOLVE-II first-in-man trial at 5 years. Cardiovascular Revascularization Medicine. 2021. doi: 10.1016/j.carrev.2021.07.017. 17. BIOSOLVE-II-Fall, GER443-012. Mit freundlicher Genehmigung von Prof. M. Haude, Rheinland Klinikum Neuss GmbH, Neuss, Deutschland 2015. 18. Torzewski J. Safety and performance of Magmaris at 48 months: BIOSOLVE-IV first cohort. Präsentiert auf: EuroPCR; 2023; ClinicalTrials.gov: NCT02817802.
BIOSOLVE-II und -IV beruhen auf einer Kaplan-Meier-Methode zur Fehlerschätzung sowie Zensierung. Die gepoolte Analyse von BIOSOLVE-II und -III beruht auf einer frequentistischen Analyse. Die 36-Monats-Daten der Analysen von BIOSOLVE-II und -III stammen aus einem Zeitraum von bis zu 1125 Tagen bei 3 Jahren.
Magmaris und BIOlute sind Marken oder eingetragene Marken der Unternehmensgruppe BIOTRONIK.
Alle anderen Marken sind Eigentum ihrer jeweiligen Inhaber.