ISAR-DESIRE 4
Randomized Clinical Trial
Intracoronary Stenting and Angiographic Results: Optimizing Treatment of Drug-Eluting Stent In-Stent Restenosis 4
Conclusion
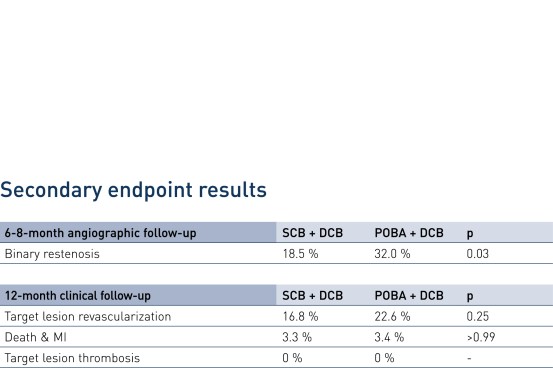
- In patients presenting with drug-eluting stent (DES) restenosis paclitaxel-coated balloon-based strategies confirmed a high clinical safety profile out to 1 year
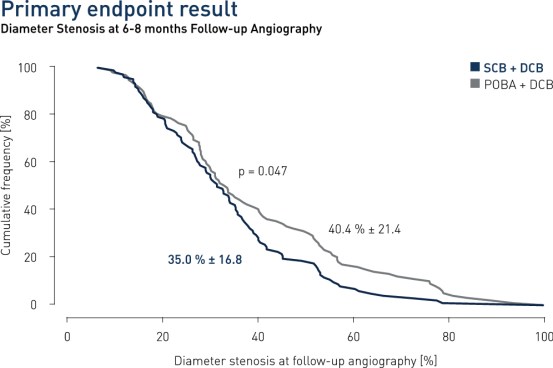
- In patients presenting with DES restenosis neointimal modification with scoring balloon significantly improves the angiographic antirestenotic efficacy of paclitaxel-coated balloon angioplasty
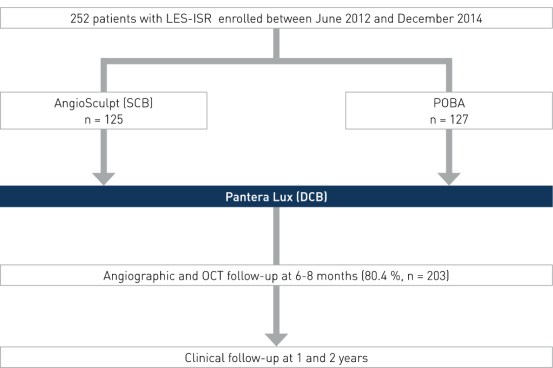
Study Design
Prospective, randomized, active, controlled multi-center clinical trial to compare the anti-restenotic efficacy of scoring balloon (SCB) pre-dilation before drug-coated balloon (DCB) therapy versus standard balloon pre-dilation (Plain Old Balloon Angioplasty, POBA) before DCB therapy in patients with limus-eluting stent (LES) restenosis. Baseline characteristics were not significantly different in the two groups.
- Number of patients (n): 252
- Principal investigator: Dr. Robert Byrne, German Heart Center, Munich, Germany
- Primary endpoint: Percent diameter stenosis (%DS) at 6-8 months
- Secondary endpoints: binary restenosis, Target Lesion Revascularization (TLR), death/myocardial infarction (MI), target lesion thrombosis
Results
Downloads
Vascular Intervention
Paclitaxel-Releasing BalloonClinically proven solution in both in-stent restenotic and de novo lesions

Vascular Intervention
Clinical StudyPaclitaxel Releasing Balloon in patients presenting with in-stent restenosis – First-in-Human
Source:
Byrne R. Presentation at TCT 2015.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
