NORDIC ICD
The NO Regular Defibrillation testing In Cardioverter Defibrillator Implantation trial
Bänsch et al., European Heart Journal 2015
Study Design
- Prospective, randomized, parallel group, multicenter, non-inferiority study 1
- Investigates the effect of defibrillation (DF) testing at the time of de novo ICD implantation on first shock efficacy during follow-up
- 1,077 (540 with and 537 without DF test) ICD and CRT-D patients at 48 centers in five countries
Key Result 1
Defibrillation (DF) testing during implantation does not improve defibrillation efficacy during follow-up, nor all-cause mortality (median 22.8 months).2
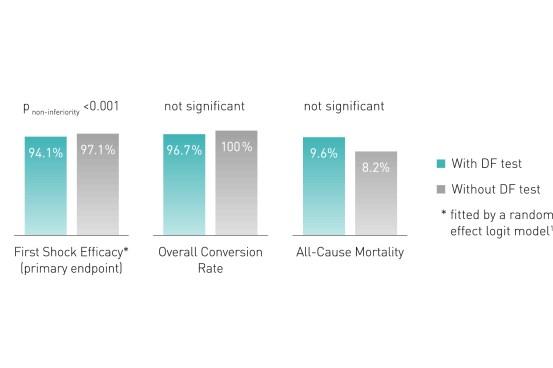
Key Result 2
DF testing, or the various measures to improve defibrillation efficacy, lengthen the procedure, and may even be harmful.2
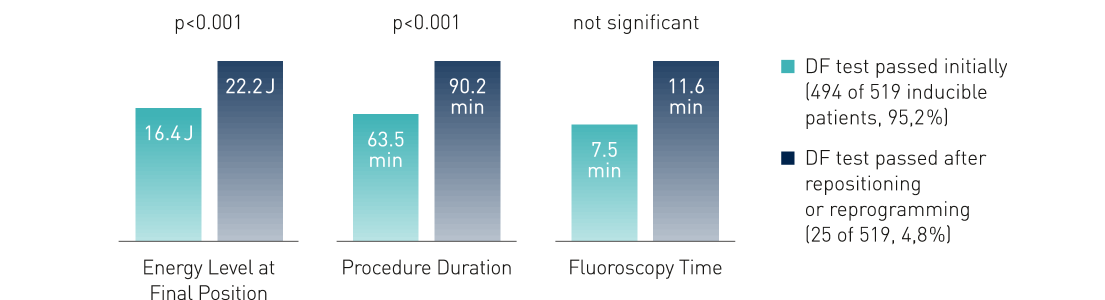
Clinical Relevance
- Results do not support the routine use of DF testing during first time ICD implantation on the left side
- Implantation strategy without DF testing should be preferred
- Results confirm the safety of omitting the DF test, which is currently routine clinical practice in many implanting centers all over the world
| Study Objective |
|
|---|---|
| 1° Endpoints |
|
| 2 ° Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Devices |
|
| Study Flowchart |
 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
1 Bänsch D, Bonnemeier H, Brandt J, Bode F, Svendsen JH, Felk A, Hauser T, Wegscheider K; The NO Regular Defibrillation testing In Cardioverter Defibrillator Implantation (NORDIC ICD) trial: concept and design of a randomized, controlled trial of intra-operative defibrillation testing during de novo defibrillator implantation, Europace, Jan 2015;17(1):142-7.
2 Bänsch D, Bonnemeier H, Brandt J, Bode F, Svendsen JH, Táborský M, Kuster S, Blomström-Lundqvist C, Felk A, Hauser T, Suling A, Wegscheider K; Intra-Operative Defibrillation Testing and Clinical Shock Efficacy in Patients with Implantable Cardioverter-Defibrillators: The NORDIC ICD Randomised Clinical Trial, European Heart Journal Jun 2015, DOI: 10.1093/eurheartj/ehv292.
