Orsiro®
Key Product Features
Product Overview
Technical Data
Media
Outstanding patient outcomes
Improving patient outcomes, year after year*
BIOFLOW-V (n = 1,334) the FDA pivotal trial1, 2, 3, 4, 5
Significant differences in TLF observed at year 1 and 2 were maintained and further increased at year 3 (8.6% vs. 14.4%, p = 0.003), driven by significant differences in TV-MI (5.5% vs. 10.1%, p = 0.004) and Ischemia driven TLR (3.4% vs. 6.9%, p = 0.008) that favor Orsiro over Xience.
TLF and components at 12, 24 and 36 Months
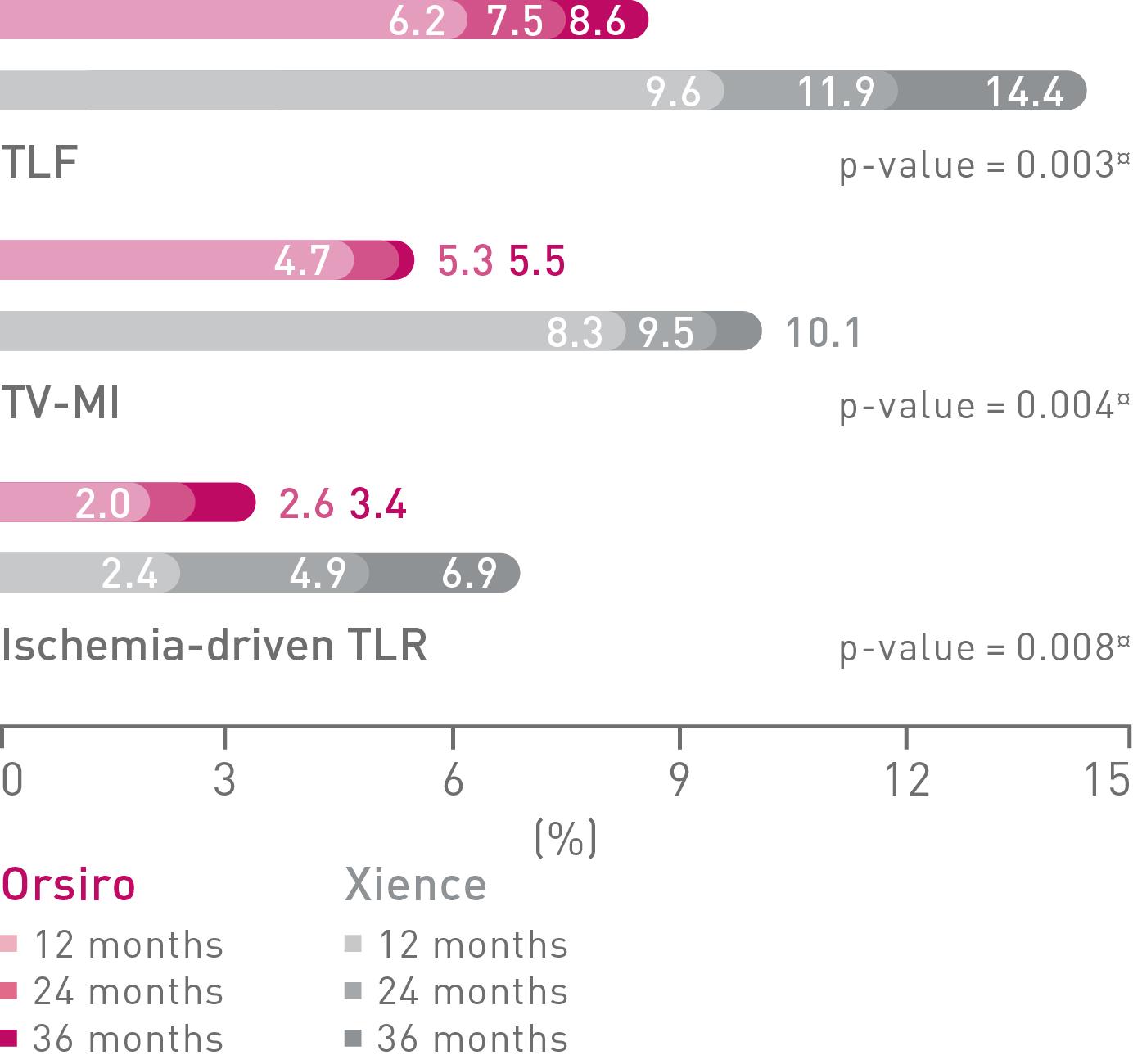
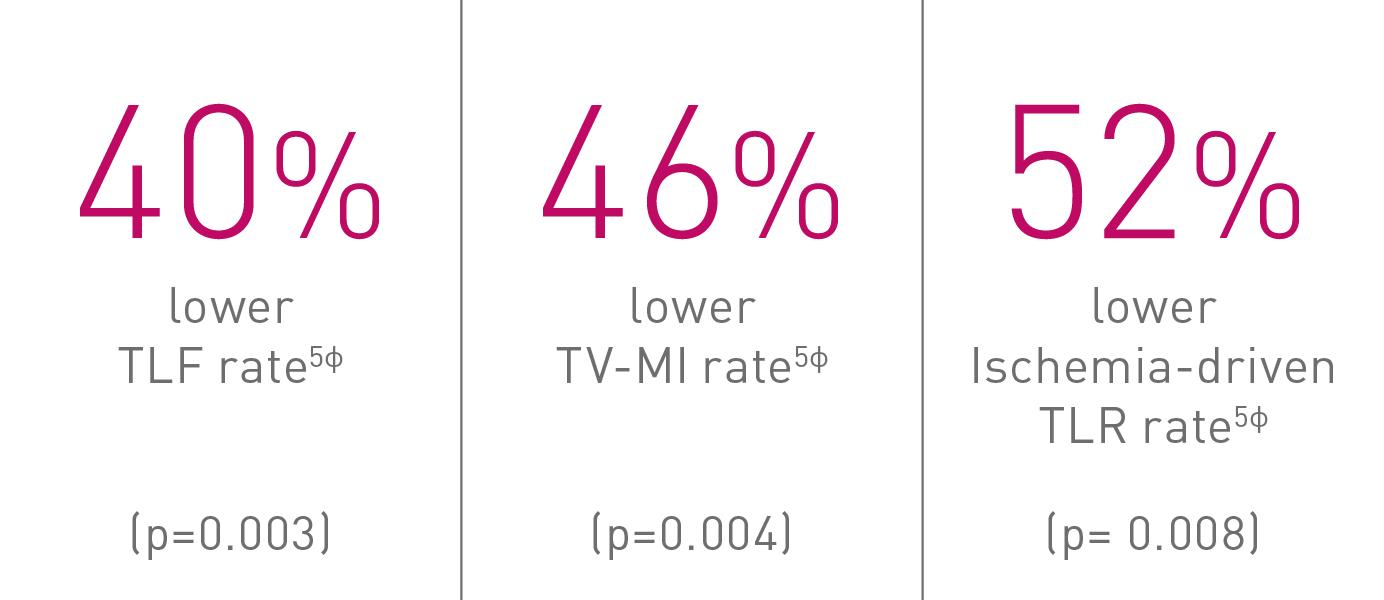
TLF – Target Lesion Failure; TV-MI – Target Vessel Myocardial Infarction; TLR – Target Lesion Revascularization. § As characterized with respect to strut thickness in Bangalore et al. Meta-analysis. ◊ Based on investigator’s interpretation of BIOFLOW-V primary endpoint results. *Compared to Xience, based on three consecutive years. ¤p-values for 36-m frequentist analysis (see supplemental material). ф vs. Xience, based on 36-m frequentist analysis (see supplemental material).
Long-term performance
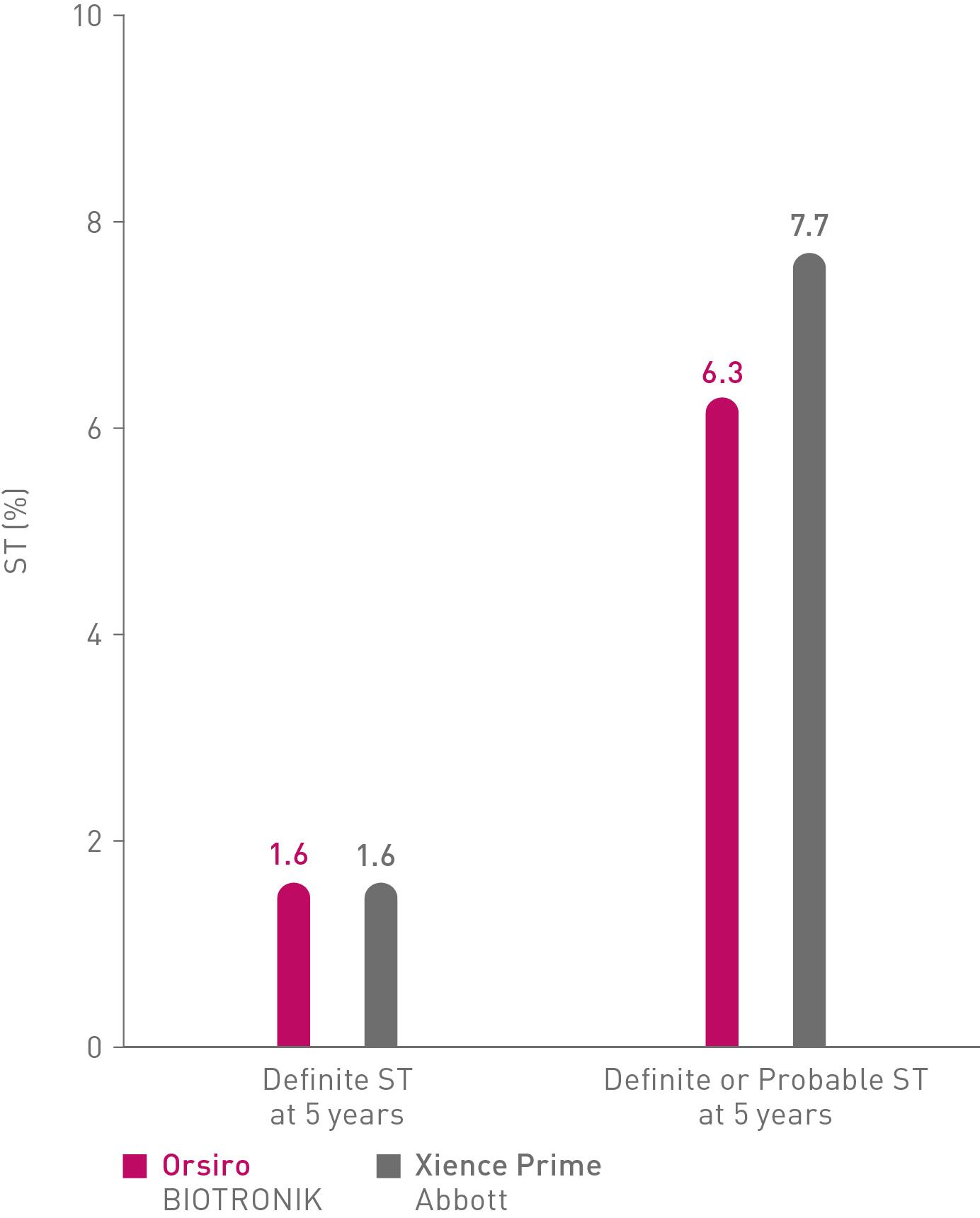
In the randomized, all-comers BIOSCIENCE trial (n= 2,119)6
Orsiro shows numerically equal or lower Stent Thrombosis (ST) in complex patients in comparison to Xience.
Excellent deliverability
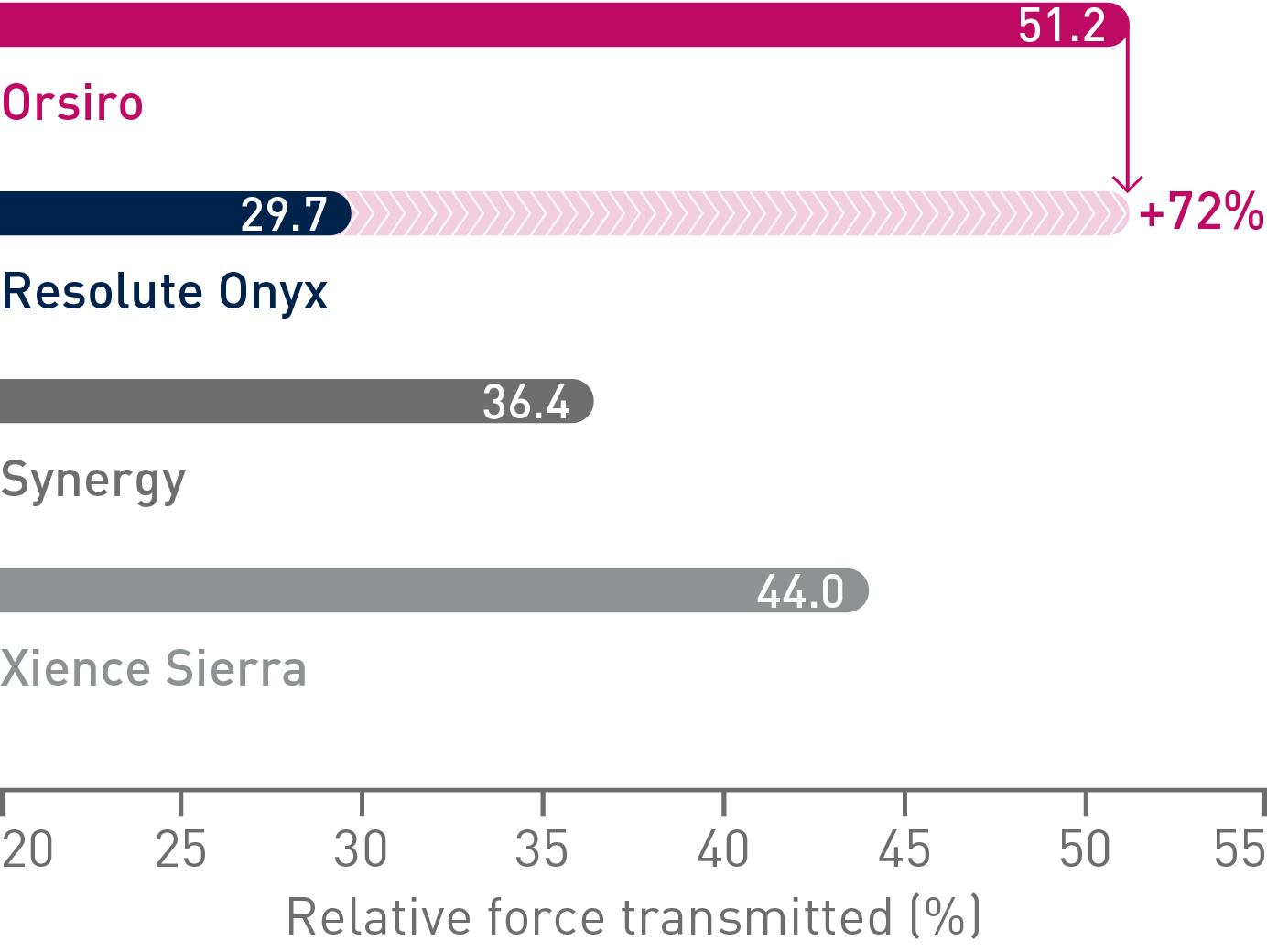
Better push
Transmits up to 72% more force from hub to tip.14
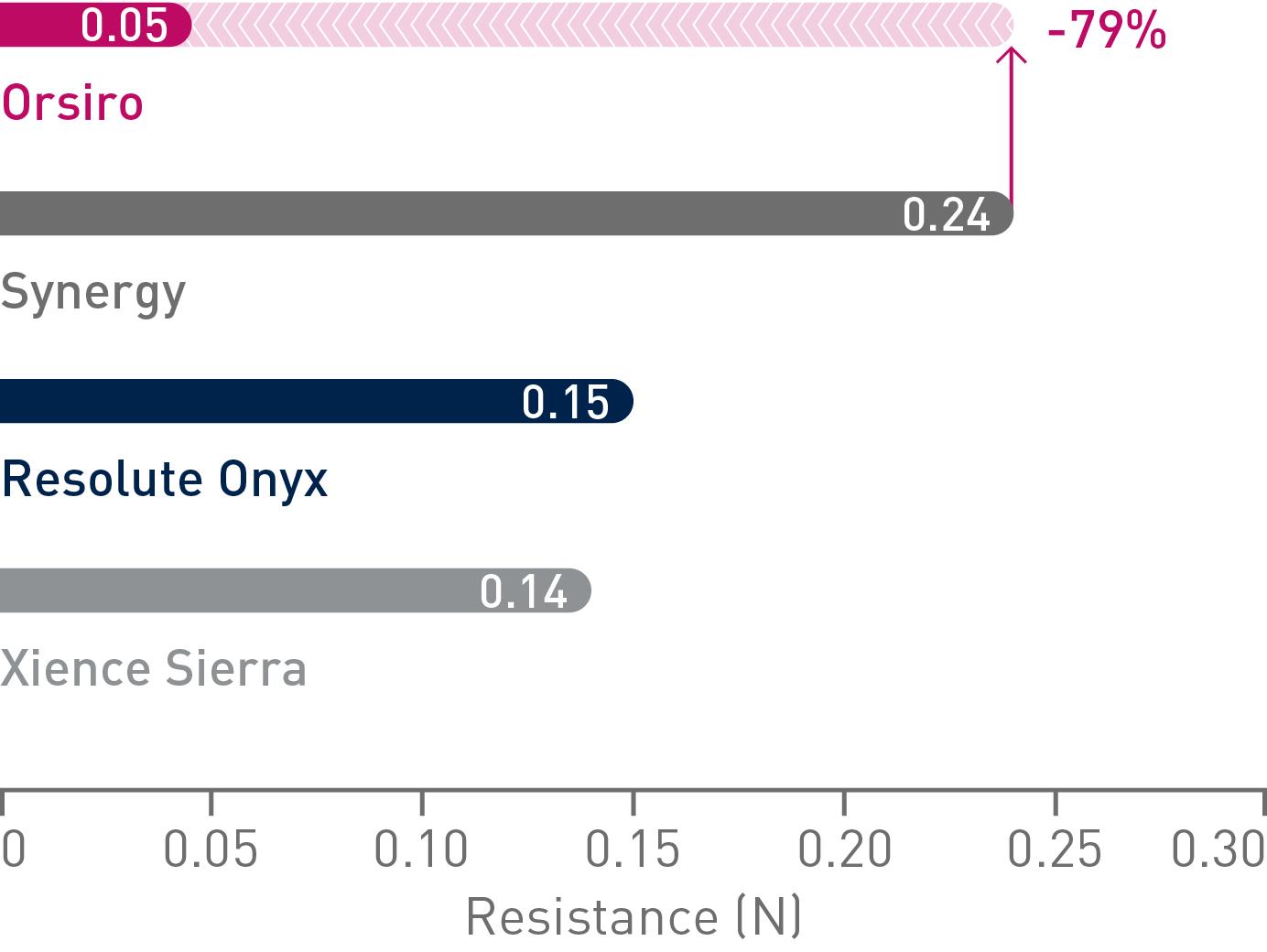
Easier cross
Up to 79% less force needed to successfully cross demanding anatomies.14

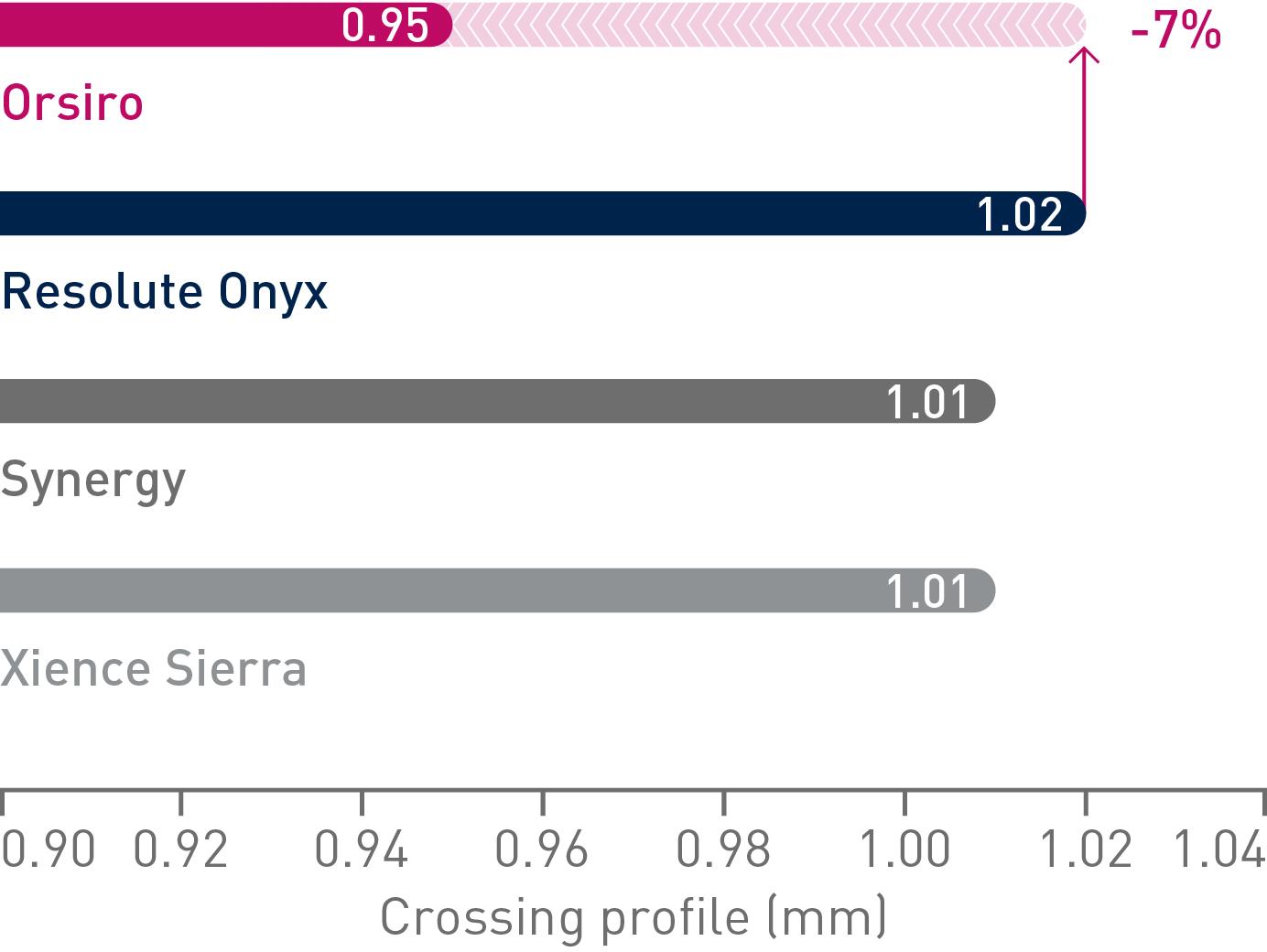
Lower crossing profile
Up to 79% less force needed to successfully cross demanding anatomies.14
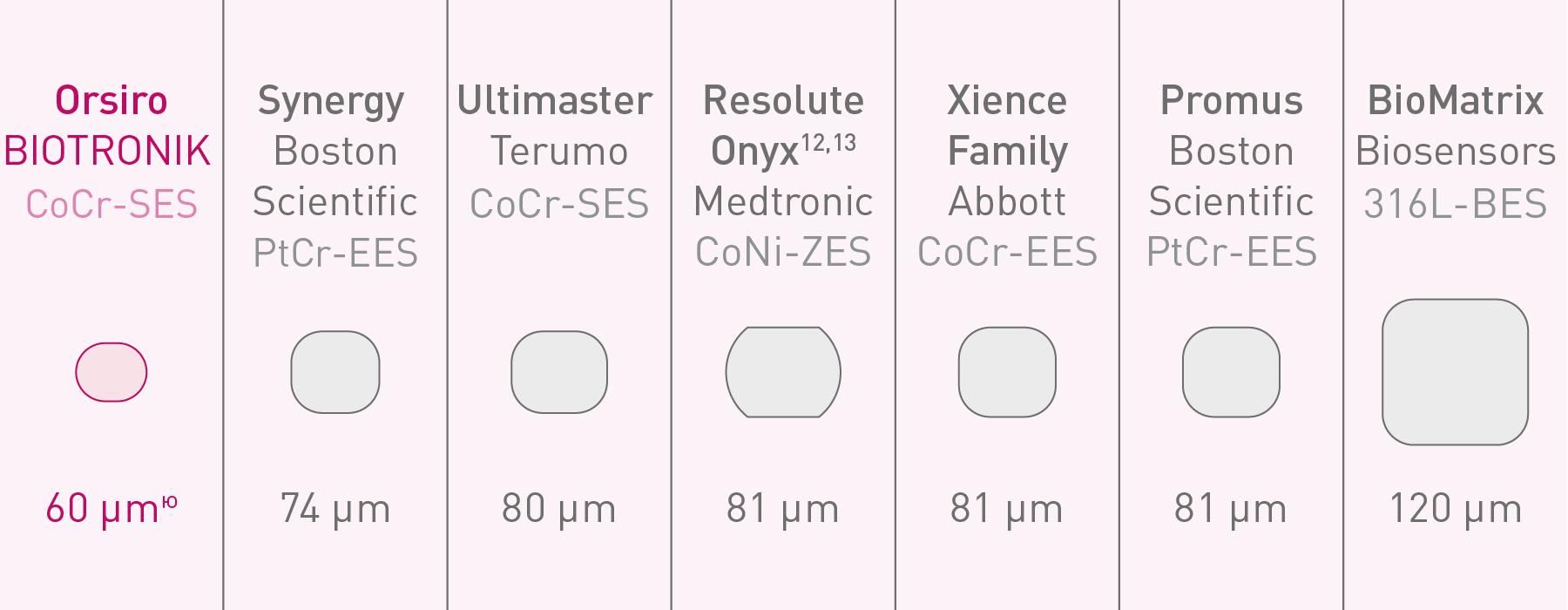
Ultrathin 60** μm struts
Improved outcomes start in the early phase
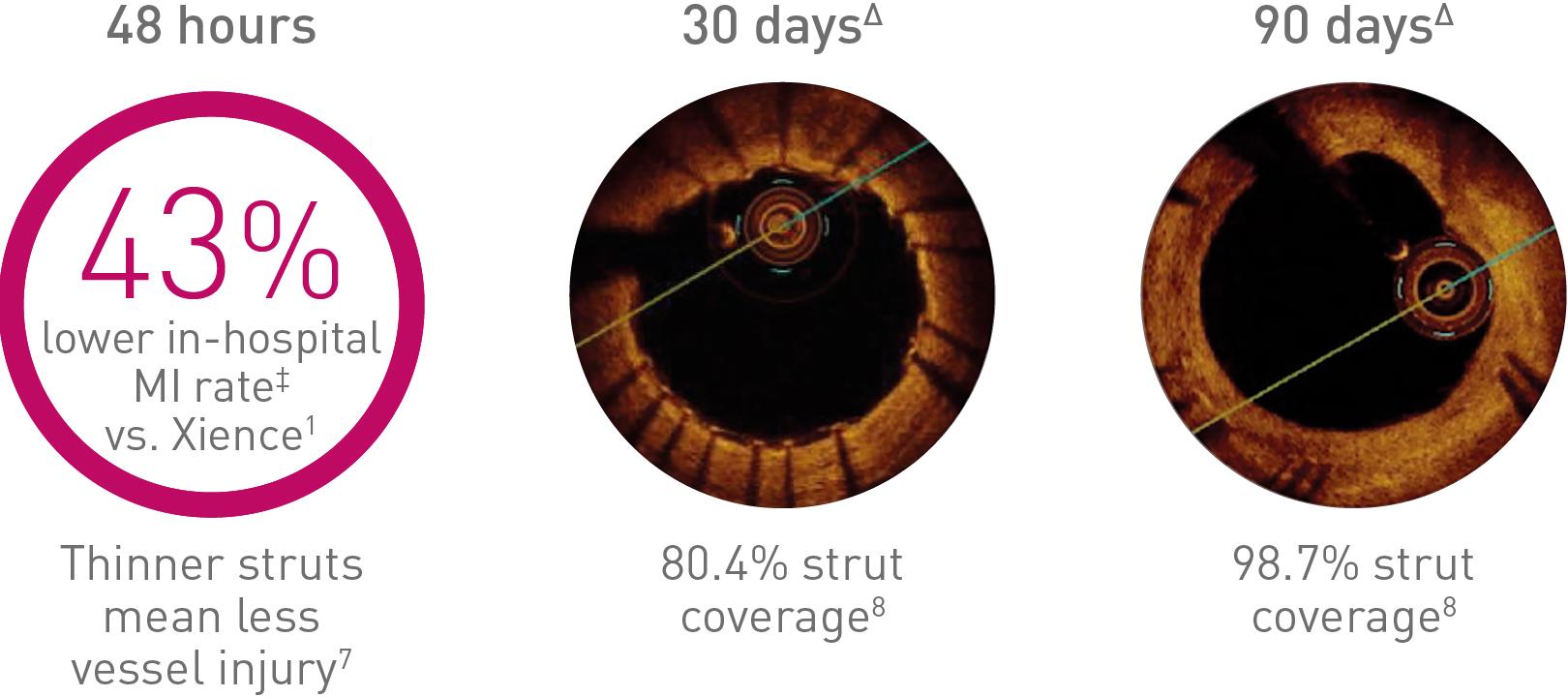
Thinner struts make the difference
Ultrathin vs. second generation DES in a large scale meta-analysis including more than 11,000 patients9, 10
16%
Relative risk reduction in TLF at 12 months RR (95% CI) 0.84 (0.72, 0.99)
‡ Driven by peri-procedural MI events (<48 hours). In-hospital rate may include events > 48 hours. Δ Images: Secco G et al. Time-related changes in neointimal tissue coverage following a new generation SES implantation: an OCT observational study. Presented at: euro PCR, May 20, 2014; Paris, France.
Strut thickness in perspective11
References
** nominal strut thickness for size ø 2.25 - 3.0mm, mean diameter 62 μm; 1. Kandzari D et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017 Oct 21; 390(10105):1843-1852; 2. Kandzari D, et al. BIOFLOW-V: A Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in the Treatment Of Subjects With up to Three De Novo or Restenotic Coronary Artery Lesions Science. Presentation at ESC 2017; 3. Kandzari D et al. Ultrathin bioresorbable polymer sirolimus-eluting stents versus thin durable polymer everolimus-eluting stents. Journal of the American College of Cardiology. 2018 Dec 17;72(25):3287-97; 4. Kandzari D et al. J Am Coll Cardiol. Cardiovasc Interven. 2020, doi: 10.1016/j. jcin.2020.02.019; 5. Kandzari D et al. J Am Coll Cardiol. Cardiovasc Interven. 2020 Supplemental Material; 6. Pilgrim T et al. 5-year outcomes of the BIOSCIENCE randomised trial. Supplementary appendix; Lancet 2018; published online Aug 28. http://dx.doi.org/10.1016/S0140-6736(18)31715-X; 7. Foin et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int J Cardiol.2014 Dec 20;177(3):800-8; 8. Secco G et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine 17.1 (2016): 38-43; 9. Bangalore S et al. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease: meta-analysis of randomized trials. Circulation. 2018 Nov 13;138(20):2216-26; 10. Bangalore S, et al. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease: meta-analysis of randomized trials. Circulation. 2018 Jul. 24: 2216-2226; 11. Stefanini GG et al. Coronary stents: novel developments. Heart. 2014 Jul 1;100(13):1051-61; 12. Low AF. Stent platform for procedural success: Introducing the Continuous Sinusoidal & Core Wire Technologies. Presented at: AsiaPCR; 22-24 January, 2015; Singapore, Singapore; 13. Tolentino A. Evolving DES Strategy: Biodegradable Polymer vs. Bioabsorbable Scaffold. Presented at: Cardiovascular Nurse/Technologist_Symposium; June 17, 2016; New York, USA; 14. BIOTRONIK data on file.
Target Lesion Failure (TLF), Target Lesion Revascularization (TLR), Target Vessel Myocardial Infarction (TV-MI), Stent Thrombosis (ST).
Orsiro, proBIO and BIOlute are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
Synergy and Promus are trademarks or registered trademarks of the Boston Scientific group of companies. Resolute and Resolute Onyx are trademarks or registered trademarks of the Medtronic group of companies. Xience, Xience Prime and Xience Sierra are trademarks or registered trademarks of the Abbott group of companies. Ultimaster is a trademark or registered trademark of the Terumo group of companies. BioMatrix is a trademark or registered trademark of the Biosensors International Group.
|
Indication |
||
|
Orsiro is indicated for improving coronary luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease, stable angina, unstable angina, non-ST-elevation myocardial infarction or documented silent ischemia due to atherosclerotic lesions in the native coronary arteries with a reference vessel diameter of 2.25 mm to 4.0 mm and a lesion length of ≤ 36 mm. |
||
|
Technical Data |
Stent |
|
|
Stent material |
Cobalt chromium, L-605 |
|
|
Passive coating |
proBIOTM (Amorphous Silicon Carbide) |
|
|
Active coating |
BIOluteTM bioabsorbable drug matrix consisting of sirolimus and polymer poly-l-lactide (PLLA) |
|
|
Nominal drug dose |
1.4 µg/mm² |
|
|
Delivery system |
||
|
Catheter type |
Fast-exchange |
|
|
Recommended guide catheter |
5F (min. I.D.Δ ≥ 0.056”) |
|
|
Guide wire diameter |
0.014” (0.36 mm) |
|
|
Usable catheter length |
140 cm |
|
|
Balloon material |
Polymer |
|
|
Coating (Distal shaft) |
Hydrophilic coating |
|
|
Marker bands |
Two platinum-iridium markers |
|
|
Proximal shaft diameter |
2.0F |
|
|
Distal shaft diameter |
ø 2.25-3.5 mm: 2.7F; ø 4.0 mm: 3.0F |
|
|
Nominal pressure (NP) |
ø 2.25-2.75, 3.5-4.0 mm: 7 atm; ø 3.0 mm: 8 atm |
|
|
Rated burst pressure (RBP) |
16atm |
|
ΔI.D. = Inner Diameter
|
Ordering Information |
Stent ø (mm) |
Stent Length (mm) |
||||||||
|
|
9 |
13 |
15 |
18 |
22 |
26 |
30 |
35 |
40 |
|
|
2.25 |
419101 |
419107 |
419113 |
419119 |
419125 |
419131 |
419137 |
|
|
|
|
2.5 |
419102 |
419108 |
419114 |
419120 |
419126 |
419132 |
419138 |
419144 |
419150 |
|
|
2.75 |
419103 |
419109 |
419115 |
419121 |
419127 |
419133 |
419139 |
419145 |
419151 |
|
|
3.0 |
419104 |
419110 |
419116 |
419122 |
419128 |
419134 |
419140 |
419146 |
419152 |
|
|
3.5 |
419105 |
419111 |
419117 |
419123 |
419129 |
419135 |
419141 |
419147 |
419153 |
|
|
4.0 |
419106 |
419112 |
419118 |
419124 |
419130 |
419136 |
419142 |
419148 |
419154 |
|