Vascular Intervention // Peripheral
Balloon-Expandable Stent System/0.035”/OTW
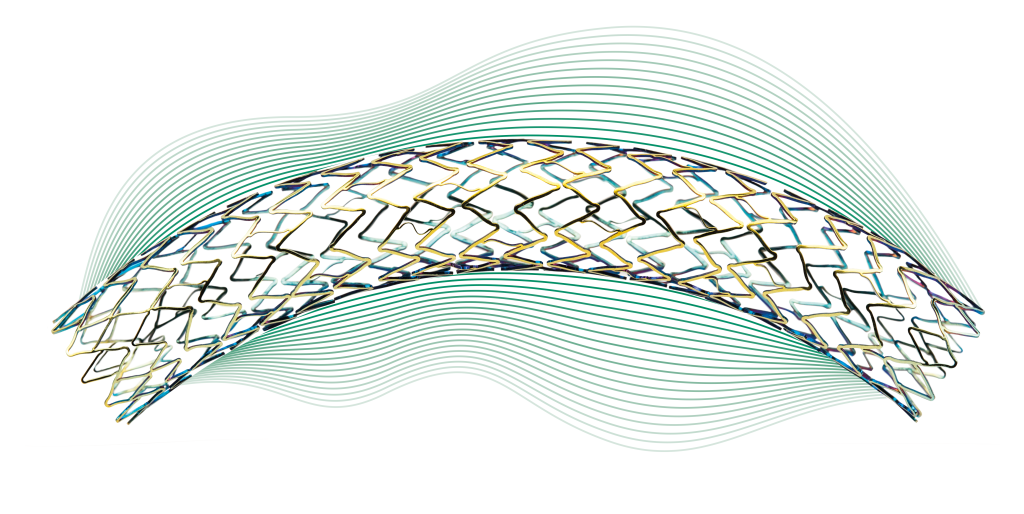
Dynamic
 Excellent trackability
Excellent trackability Stent designed for flexibility in iliac arteries
Stent designed for flexibility in iliac arteries Improved stent surface biocompatibility
Improved stent surface biocompatibility
Excellent trackability
The Dynamic delivery system offers great trackability compared to competitive balloon expandable devices.1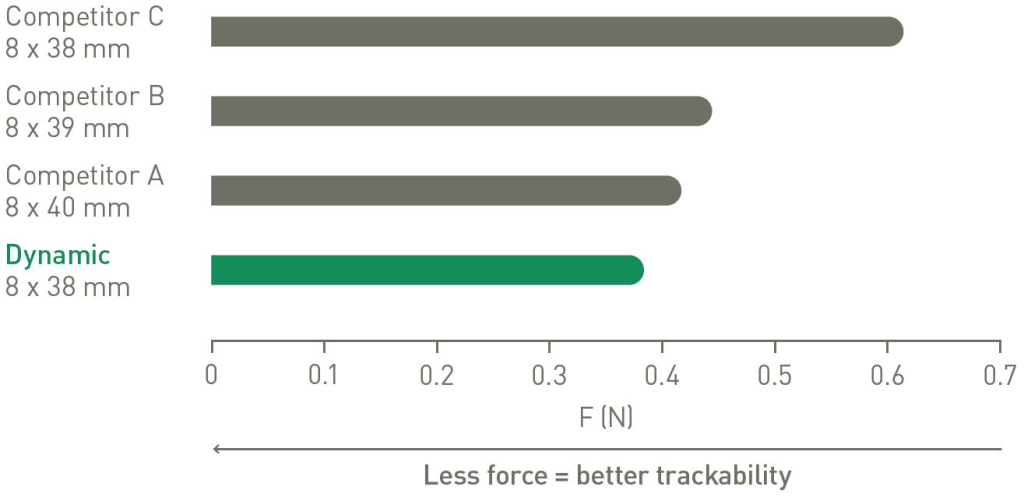
Stent designed for flexibility in iliac arteries
The helical stent design provides optimal flexibility respecting vessel movement.
Peak-to-Valley design avoiding any fish-scaling effects and optimizing stent scaffolding.

Smooth tapered tip
Dynamic has a colored tip for better visibility allowing easy guide wire insertion. The smooth tapered tip design facilitates trackability in tortuous anatomy.
Improved stent surface biocompatibility2
TheproBIOsilicon carbide coating acts as a barrier between the metal stent and the surrounding tissue and blood, protecting the surface of the stent.
By providing a barrier against ion release, the coating creates a surface that reduces platelet aggregation while facilitating endothelialization.2
 Dynamic
Dynamic
Indicated for the treatment of de novo or restenotic atherosclerotic lesions in iliac arteries.3
Technical Data
| Dynamic Stent | |
|---|---|
| Stent | Balloon-expandable |
| Stent material | Stainless steel |
| Strut thickness | 160 μm (ø 5.0 - 8.0 mm) 180 μm (ø 9.0 - 10.0 mm) |
| Shortening | Negligible |
| Stent coating | proBIO (Amorphous Silicon Carbide) |
| Sizes | ø 5.0 - 10.0 mm; L: 15 - 25 - 38 - 56 mm |
| Delivery System | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.035" |
| Tip | Soft, short, tapered, colored |
| Balloon markers | 2 swaged markers |
| Shaft | 5 F, hydrophobic coating, dual-lumen |
| Usable length | 80 and 130 cm (ø 5.0 - 8.0 mm) |
| Markers | 2 swaged markers |
| Guide wire lumen | Hydrophobic coating |
| Nominal pressure (NP) | 9 atm |
| Rated burst pressure (RBP) | 15 atm (ø 5.0 - 8.0 mm) 13 atm (ø 9.0 - 10.0 mm) |
Ordering Information
| Stent ø (mm) | Catheter length 80 cm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.0 | 350110 (5F) | 350114 (5F) | 350120 (6F) | 350126 (6F) | |||||||||
| 6.0 (6F) | 350111 | 350115 | 350121 | 350127 | |||||||||
| 7.0 (6F) | 350112 | 350116 | 350122 | 350128 | |||||||||
| 8.0 (6F) | 350113 | 350117 | 350123 | 350129 | |||||||||
| 9.0 (6F) | - | 350118 | 350124 | 350130 | |||||||||
| 10.0 (7F) | - | 350119 | 350125 | 350131 | |||||||||
| Stent ø (mm) | Catheter length 130 cm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.0 | 350132 (5F) | 350136 (5F) | 350140 (6F) | 350144 (6F) | |||||||||
| 6.0 (6F) | 350133 | 350137 | 350141 | 350145 | |||||||||
| 7.0 (6F) | 350134 | 350138 | 350142 | 350146 | |||||||||
| 8.0 (6F) | 350135 | 350139 | 350143 | 350147 | |||||||||
1 BIOTRONIK data on file;
2 Rzany A, Schaldach M. Smart Material Silicon Carbide: Reduced Activation of Cells and Proteins on a-SiC:H-coated Stainless Steel. Progress in Biomedical Research 2001; May: 182- 194;
3 Australia: not TGA approved for use within common iliac arteries.

