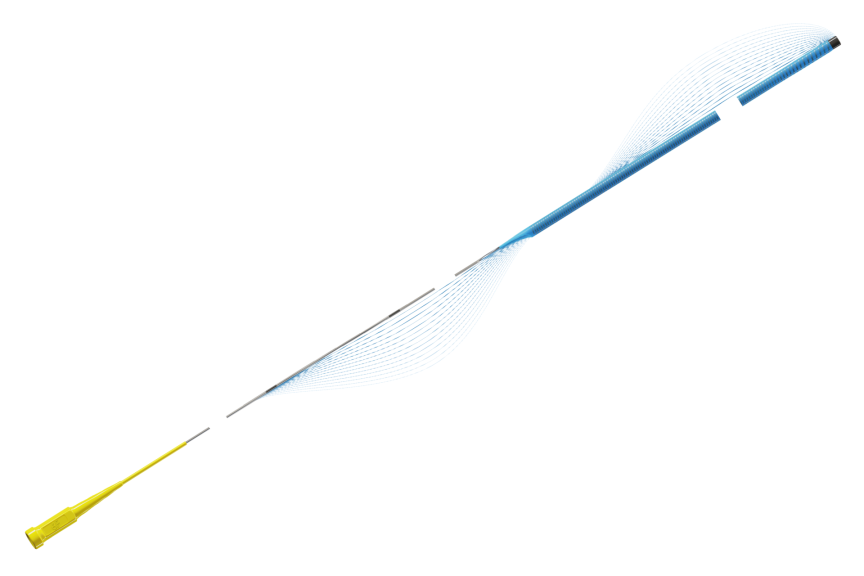
Guidion Hydro
Designed for reliable delivery of devices to target
Guidion Hydro guide extension catheter has a radiopaque soft tip for true distal end visibility facilitating precise device positioning. The high density coiling of the distal shaft ensures optimal lumen integrity and smooth device delivery. A proximal oval push rod design keeps the profile low while securing optimal pushability.1
Product Highlights
Image
True distal end visibility¹
For precise device positioning
Image
Flex Zoneᵃ and hydrophilic coating
For advanced trackability1
Image
Coil reinforced shaft
For smooth device delivery1
Product Overview
Guidion Hydro
Image

Radiopaque soft tip
Flex Zone
Coil reinforced shaft
Transition Zone
Oval push rod
Technical Data
| Guide Extension Catheter | |
|---|---|
| Distal shaft length | 25 cm |
| Shaft material | Polyether Block Amide |
| Tip | Flexible, radiopaque, 1.3 mm length |
| Outer coating | Hydrophilic distal 10 cm |
| Exit markers | 95 cm and 105 cm |
| Usable length | 150 cm |
Ordering Information
| Ordering number | IMDS article number | Required guide catheter ID | Guidion Hydro ID |
|---|---|---|---|
| 451663 | G50F25150 | 5F, ID ≥ 0.056" (1.42 mm) | 0.041" (1.04 mm) |
| 451664 | G60F25150 | 6F, ID ≥ 0.070" (1.78 mm) | 0.056" (1.42 mm) |
| 451665 | G70F25150 | 7F, ID ≥ 0.078" (1.98 mm) | 0.062" (1.57 mm) |
| 451666 | G80F25150 | 8F, ID ≥ 0.088" (2.24 mm) | 0.071" (1.80 mm) |
Downloads and Related Links
Downloads
Related Links
How can we help you?
References
a. 6 and 7F.
1. IMDS Data on File.
Guidion Hydro is a trademark of IMDS.
Manufactured by IMDS and distributed by BIOTRONIK in selected countries.