Vascular Intervention // Coronary
Covered Coronary Stent System
PK Papyrus

 Covered single stent design
Covered single stent design 58% greater flexibility
58% greater flexibility Low crossing profile
Low crossing profile
Covered single stent design
With its covered single stent design, PK Papyrus achieves greater bending flexibility and a smaller crossing profile compared to the traditional sandwich design stent1, allowing you to seal perforations with confidence.

58% greater flexibility1
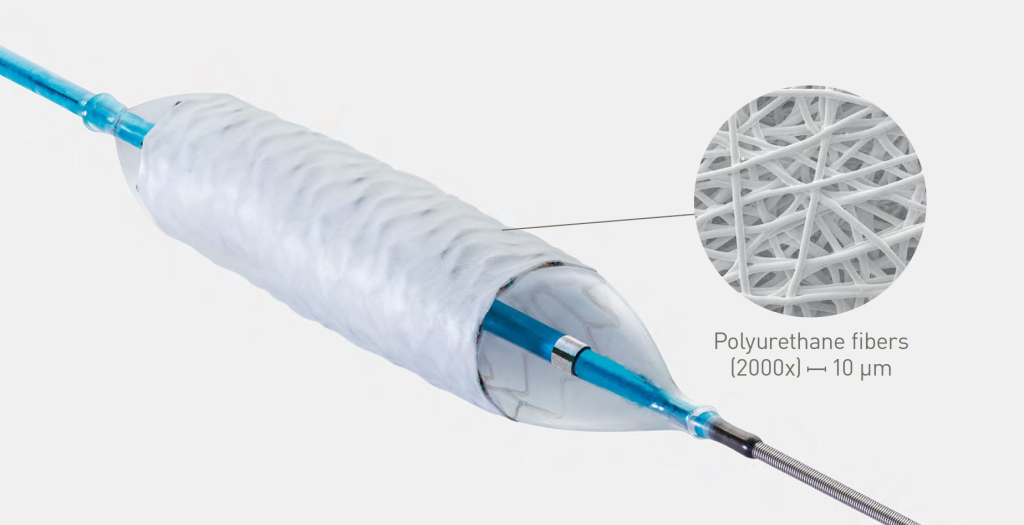
Innovative polyurethane membrane Enabled by an electrospinning process, creating a polyurethane membrane only 90 μm thin.
Enabled by an electrospinning process, creating a polyurethane membrane only 90 μm thin.
Low crossing profile

 PK Papyrus
PK PapyrusIndicated for acute coronary artery perforations.*
Media
PK Papyrus
Technical Data
| Stent | |
|---|---|
| Stent cover material | Non-woven, electrospun polyurethane |
| Stent cover thickness | 90 μm |
| Stent strut thickness | ø 2.5 - 3.0 mm: 60 μm (0.0024"); ø 3.5 - 4.0 mm: 80 μm (0.0031"); ø 4.5 - 5.0 mm: 120 μm (0.0047") |
| Stent material | Cobalt chromium (L-605) with proBIO amorphous silicon carbide coating |
| Maximum stent expansion diameter | ø 2.5 - 3.0 mm: 3.50 mm; ø 3.5 - 4.0 mm: 4.65 mm; ø 4.5 - 5.0 mm: 5.63 mm |
| Delivery System | |
|---|---|
| Guide wire diameter | 0.014" |
| Usable catheter length | 140 cm |
| Recommended guide catheter | ø 2.5 - 4.0 mm: 5F (min. I.D.** 0.056"); ø 4.5 - 5.0 mm: 6F (min. I.D.** 0.070") |
| Nominal pressure (NP) | ø 2.5 - 3.5 mm: 8 atm; ø 4.0 - 5.0 mm: 7 atm |
| Rated burst pressure (RBP) | ø 2.5 - 4.0 mm: 16 atm; ø 4.5 - 5.0 mm: 14 atm |
| **I.D. = Inner Diameter | |
Ordering Information
| 2.5 (5F) | 369380 | 369386 | - | |||||||||||
| 3.0 (5F) | 369381 | 369387 | 381789 | |||||||||||
| 3.5 (5F) | 369382 | 369388 | 381790 | |||||||||||
| 4.0 (5F) | 369383 | 369389 | 381791 | |||||||||||
| 4.5 (6F) | 369384 | 369390 | 369392 | |||||||||||
| 5.0 (6F) | 369385 | 369391 | 369393 | |||||||||||
Contact
1 Compared to Jostent Graftmaster 3.0/16 (BIOTRONIK data on file);
2 Compared to Graftmaster 2.8/16 (BIOTRONIK data on file).
Jostent and Graftmaster are registered trademarks of Abbott.
*Indication as per IFU.

