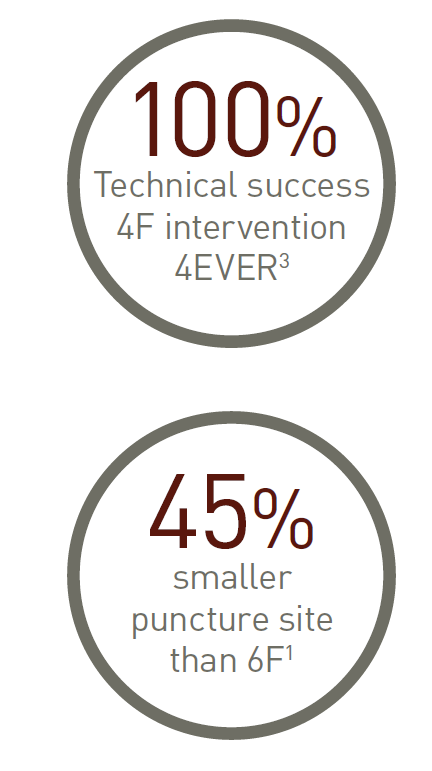
Self-Expanding Stent System/0.018”/OTW
A unique combination of 3 technologies

Pulsar 18 T3
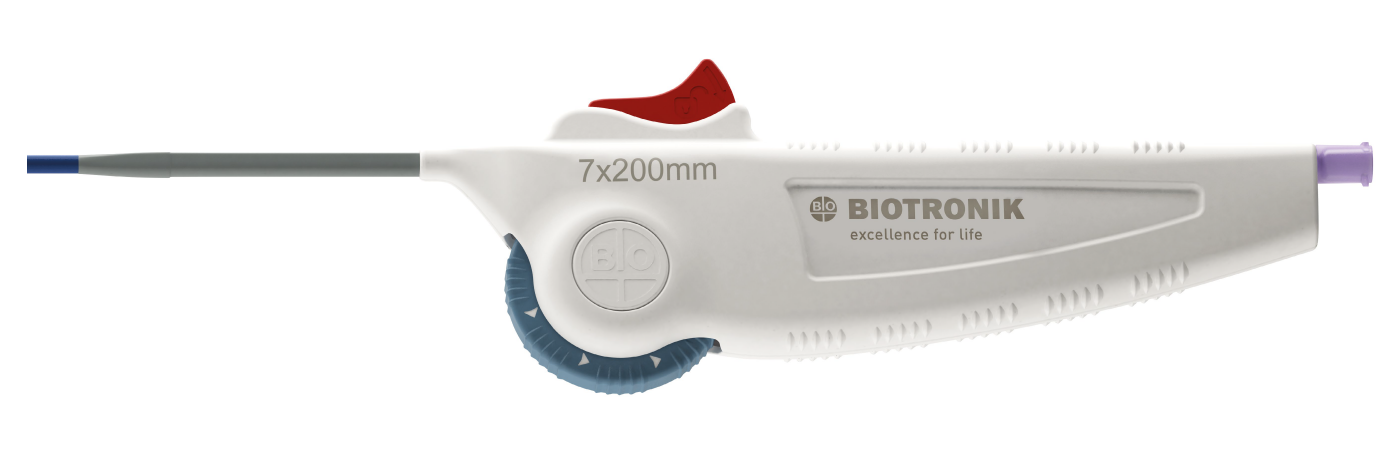
Easy to use, intuitive wheel-operated handle and one-handed deployment
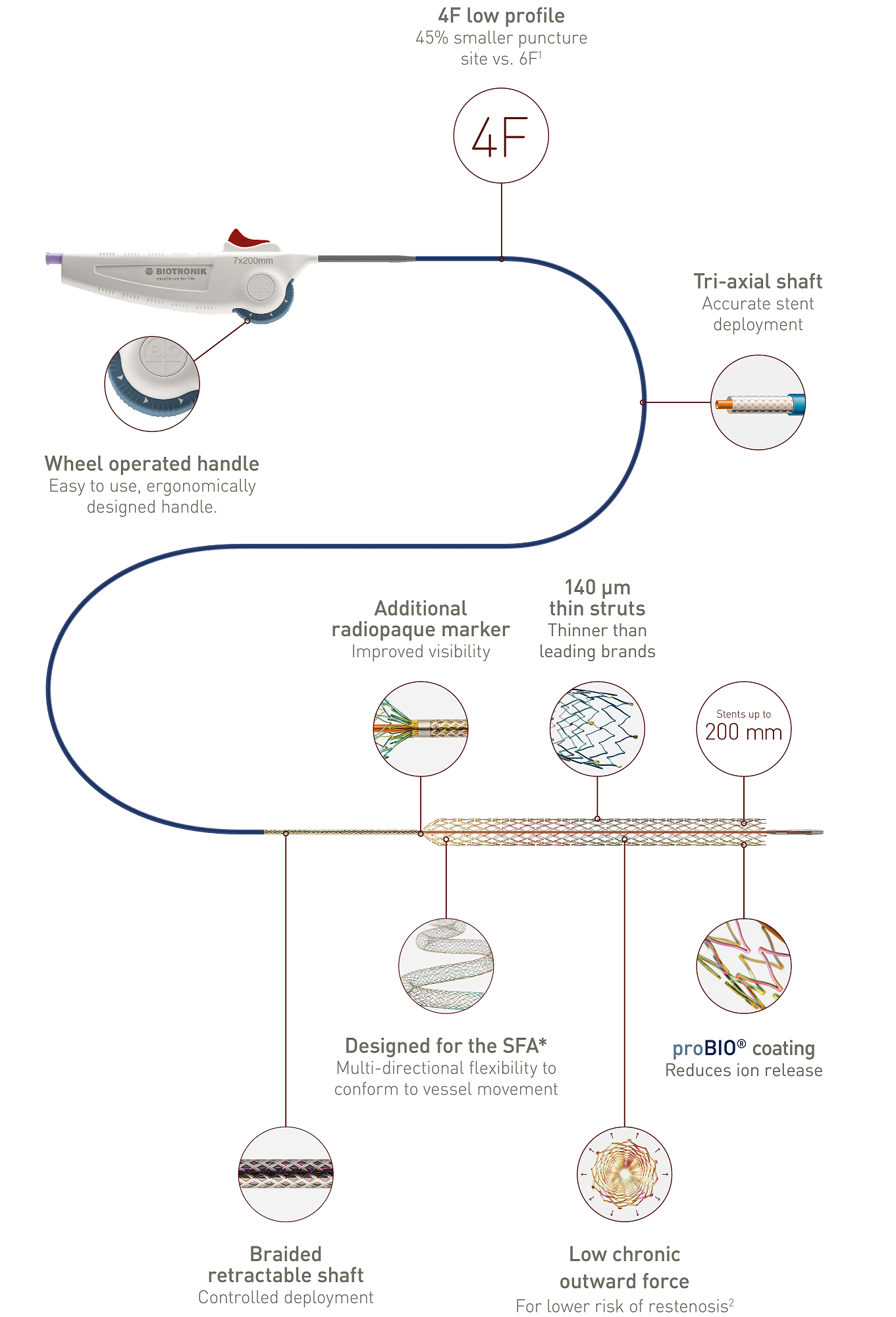
Unique tri-axial shaft design on a 4F low-profile delivery system
Wheel-operated handle ergonomically designed
Easy-to-use handle
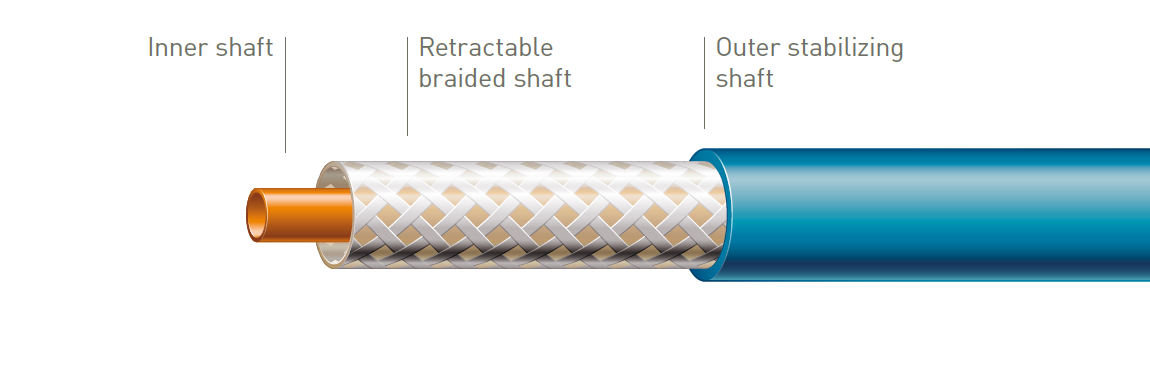
Tri-axial system with braided retractable shaft
Accurate stent deployment
The outer stabilizing shaft isolates the retractable shaft from friction caused by the introducer valve to ensure accurate stent deployment.

Thin struts and low chronic outward force
140 μm thin struts - thinner than leading brands4
Thinner struts for lower chronic outward force (COF)5
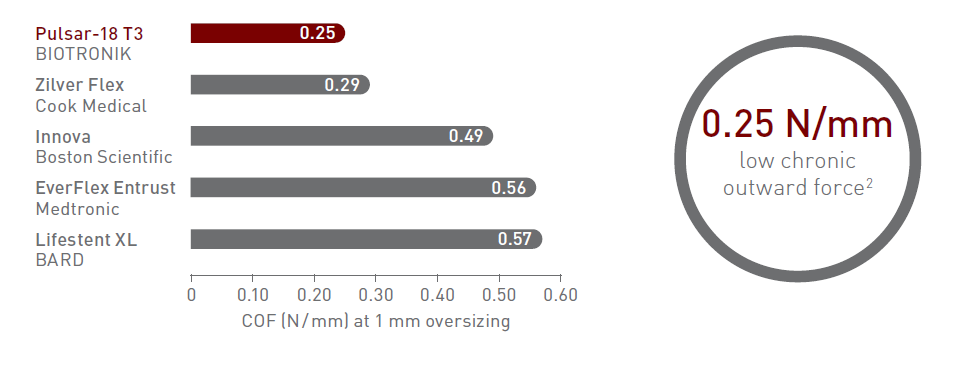
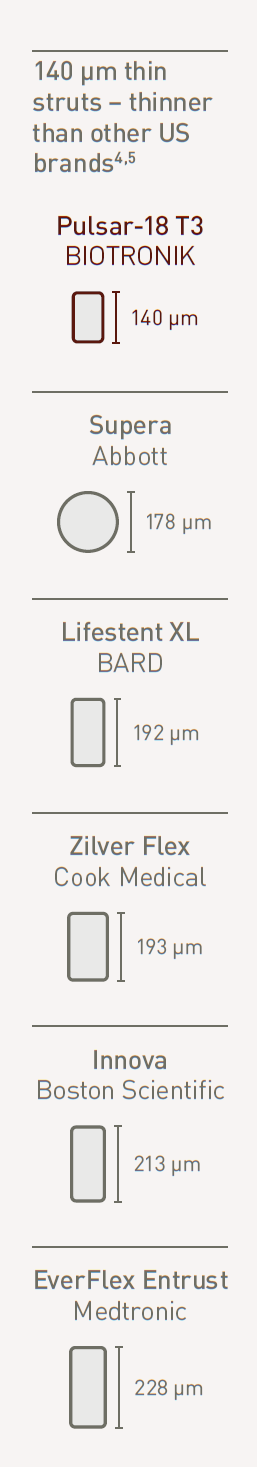
Thinner struts and lower COF make a difference:*
- Lower risk of restenosis2
- Reduced vessel injury and inflammation2
- Faster endothelialization6,7
*As demonstrated in pre-clinical studies
Vessel response on SE stent 1 mm oversizing showing neointimal hyperplasia at 90 days8*

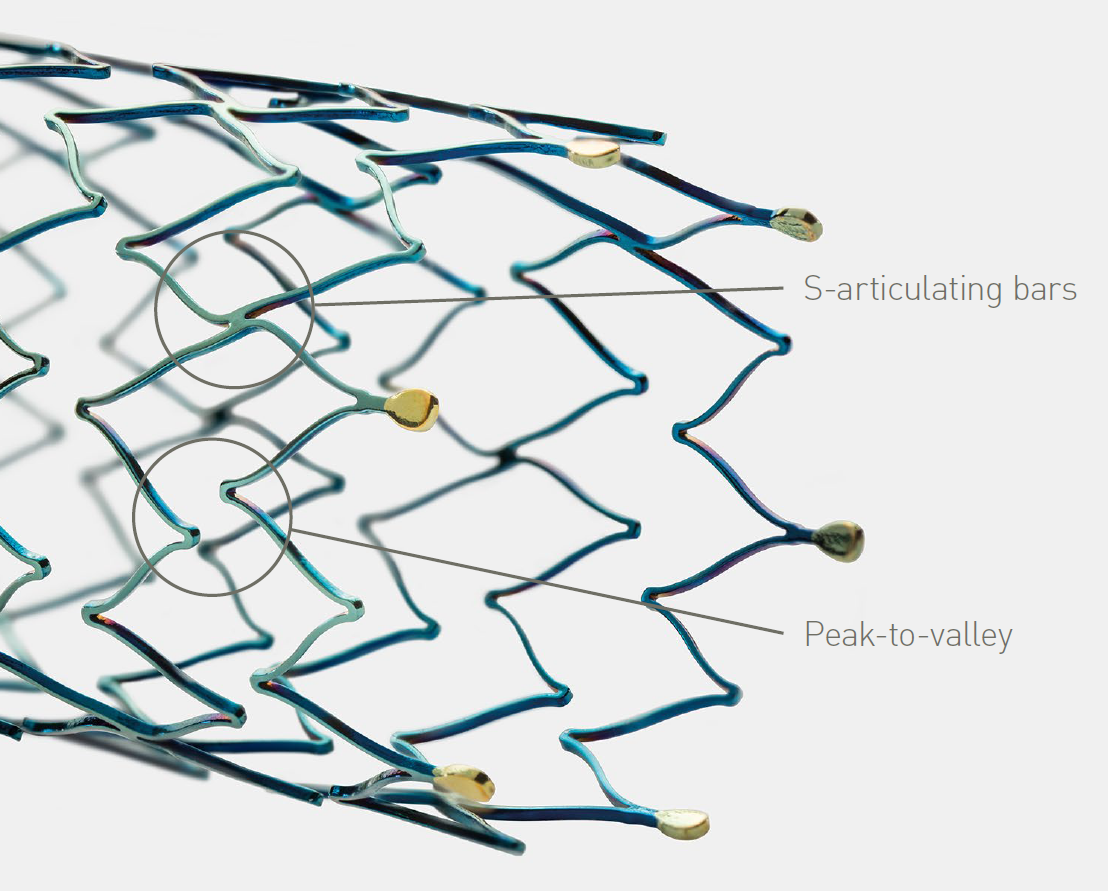
Sufficient radial force for long term vessel support, even in calcified lesions
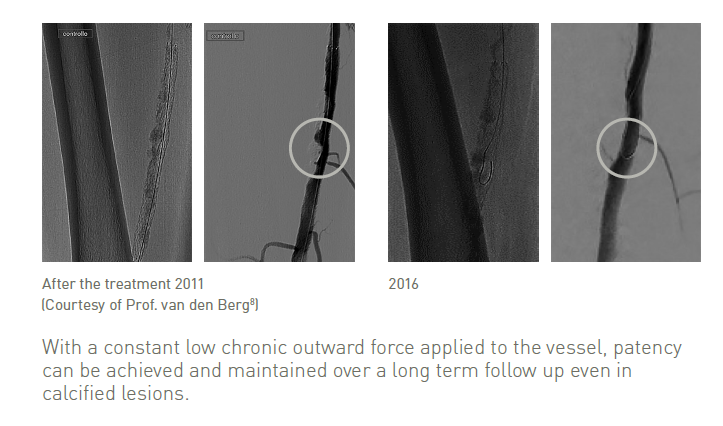

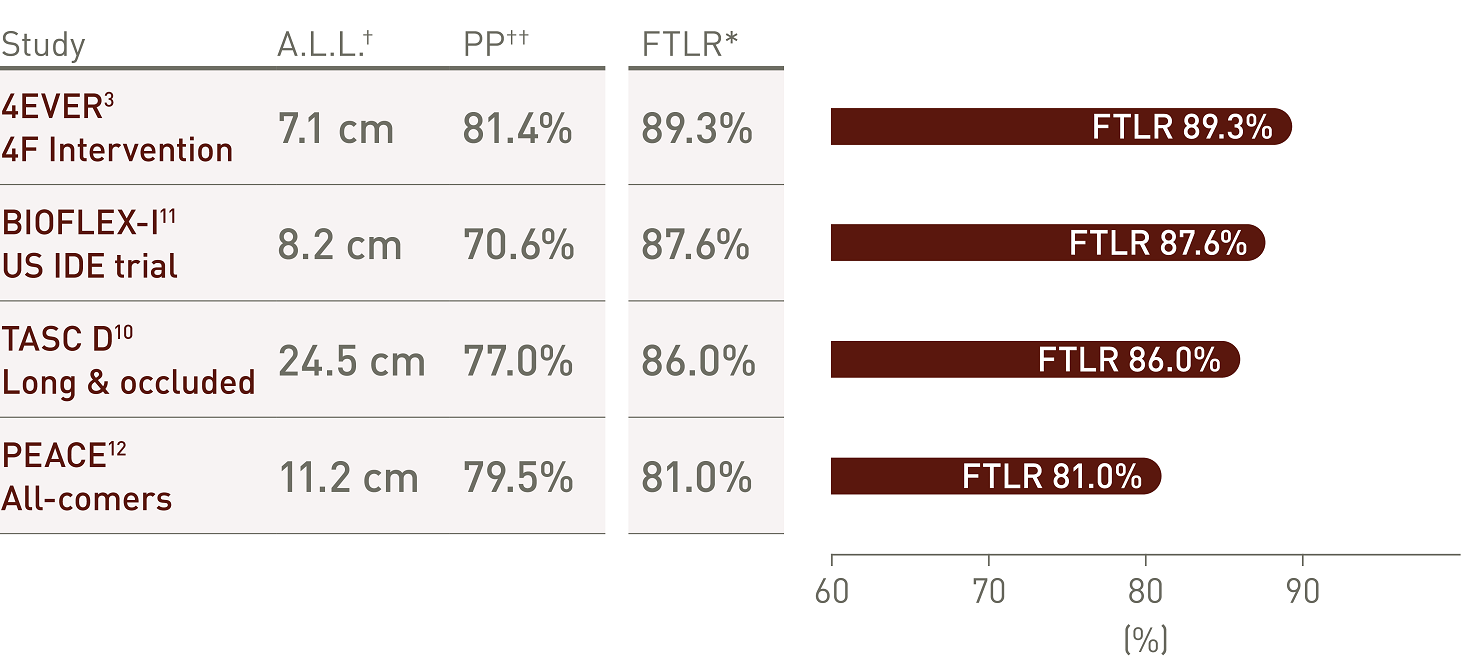
Clinically proven thin struts stent design
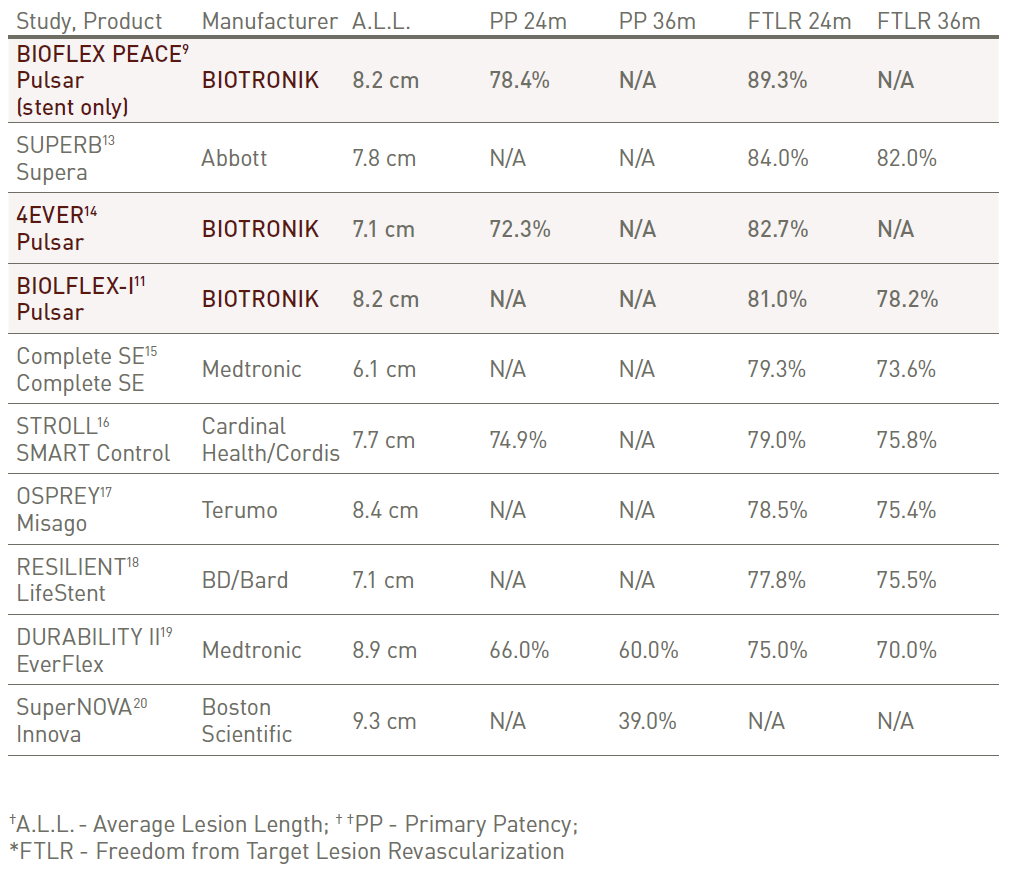
Long-term outcomes in perspective
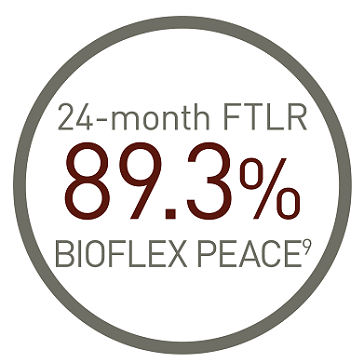
Pulsar stent outcomes at 24 and 36 months, highlighting long-term safety and efficacy.
Pulsar-18 T3
Technical Data
| Pulsar-18 T3 Stent | |
|---|---|
| Catheter type |
OTW |
| Recommended guide wire |
0.018" |
| Stent material |
Nitinol |
| Strut thickness |
140 μm |
| Strut width |
85 μm - 90 μm |
| Stent coating |
proBIO®️(Amorphous Silicon Carbide) |
| Stent markers |
6 gold markers each end |
| Sizes |
ø 4.0 - 7.0 mm; L: 20 - 200 mm |
| Shaft |
4F, hydrophobic coating, tri-axial |
| Usable length |
90 cm and 135 cm |
Ordering Information
| Stent ø (mm) | Catheter length 90 cm (Stent length mm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | ||
| 4.0(4F) | 430437 | 430438 | 430439 | 430440 | 430441 | 430442 | 430443 | 430444 | 430445 | 430446 | |
| 5.0(4F) | 430447 | 430448 | 430449 | 430450 | 430451 | 430452 | 430453 | 430454 | 430455 | 430456 | |
| 6.0(4F) | 430457 | 430458 | 430459 | 430460 | 430461 | 430462 | 430463 | 430464 | 430465 | 430466 | |
| 7.0(4F) | 430467 | 430468 | 430469 | 430470 | 430471 | 430472 | 430473 | 430474 | 430475 | 430476 | |
| Stent ø (mm) | Catheter length 135 cm (Stent length mm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | ||
| 4.0(4F) | 430477 | 430478 | 430479 | 430480 | 430481 | 430482 | 430483 | 430484 | 430485 | 430486 | |
| 5.0(4F) | 430487 | 430488 | 430489 | 430490 | 430491 | 430492 | 430493 | 430494 | 430495 | 430496 | |
| 6.0(4F) | 430497 | 430498 | 430499 | 430500 | 430501 | 430502 | 430503 | 430504 | 430505 | 430506 | |
| 7.0(4F) | 430507 | 430508 | 430509 | 430510 | 430511 | 430512 | 430513 | 430514 | 430515 | 430516 | |
Contact
1. BIOTRONIK data on file;
2. Zhao HQ Late stent expansion and neointimal proliferation of oversized nitinol stents in
peripheral arteries. Cardiovasc. Interv. Radiol. 2009; 32(4); 720-6;
3. Bosiers M et al. 4-French – compatible endovascular material is safe & effective in the treatment of femoropopliteal occlusive disease: Results of the 4EVER Trial. ENDOVASC THER 2013; 20: 746-756;
4. BIOTRONIK data on file. 6.0 mm diameters;
5. BIOTRONIK data on file. 6.0 mm diameters. Supera stent not possible to test due to its design and applied test method;
6. Koskinas C. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. JACC 2012 10;59(15):1337-49;
7. Koppara T. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ Cardiovasc Interv. 2015 8(6):e002427;
8. Funovics M. Correlation between chronic outward force (COF) and neointimal hyperplasia in self-expanding nitinol stents in swine in clinically relevant oversizing ranges. Presented at: LINC, Jan 26, 2017; Leipzig, Germany;
9. Lichtenberg et al. Effectiveness of the Pulsar-18 self-expanding stent with optional drug-coated balloon angioplasty in the treatment of femoropopliteal lesions - the BIOFLEX PEACE All-Comers Registry. Vasa (2019), 1-9. doi_10.1024/0301-1526/a000785;
10. Lichtenberg M.Superficial Femoral Artery TASC D registry: 12-month effectiveness analysis of the Pulsar-18 SE nitinol stent in patients with critical limb ischemia. J Cardiovasc Surg (Torino). 2013 ; 54(4):433-9;
11. BIOFLEX-I Pulsar 2018 Post Approval Clinical Report_Final_36m;
12. Lichtenberg M. et al PEACE I All-Comers Registry: Patency Evaluation After Implantation of the 4-French Pulsar-18 Self-Expanding Nitinol Stent in Femoropopliteal Lesions. J ENDOVASC THER. 2014;21:373–380, doi:10.1583/13-4637R.1;
13. Garcia LA et al. SUPERB Final 3-Year Outcomes Using Interwoven Nitinol Biometric Supera Stent. Catherization and Cardiovascular Interventions 2017; 89: 1259-1267;
14. Bosiers M. 4EVER 24 month results: long-term results of 4F Pulsar stent in femoropopliteal lesions. Presented at: CIRSE 2013; Barcelona, Spain;
15. Medtronic Complete SE SSED P110040 (September 19, 2013);
16. Bunte M et al. in STROLL Catheterization and Cardiovascular Interventions 2018; 92:106-114;
17. Osprey Misago P140002 (May 22, 2015);
18. Laird J et al. RESILIENT SFA nitinol stenting JET 2012;19:1-9;
19. Rocha-Singh et al. DURABILITY II Three-Year Follow-up. Catheterization and Cardiovascular Interventions 2015; 86:164-170;
20. SuperNOVA. US Food and Drug Administration, Center for Devices and Radiological Health, Innova™ Vascular Self-Expanding Stent System P140028.
Leading competitors have been selected based on the PV Stent Revenue Market Shares EU, 2017 and PV Revenue Market Shares APAC 2015; (Source: Millennium Research Group Inc.). Latest SFA self expanding stents for each manufacturer; Zilver and Zilver Flex are trademarks or registered trademarks of Cook Medical Technologies or its affiliates. Innova is a trademark or registered trademark of Boston Scientific or its affiliates. Everflex and Entrust are trademarks or registered trademarks of Medtronic or its affiliates. Lifestent is a trademark or registered trademark of C. R. Bard or its affiliates. Supera is a trademark or registered trademark of the Abbott Group of Companies. S.M.A.R.T. Control is a trademark or registered trademark of Cardinal Health or its affiliates.
*Indication as per IFU.
Pulsar and proBIO®️are trademarks or registered trademarks of the BIOTRONIK Group of Companies.;