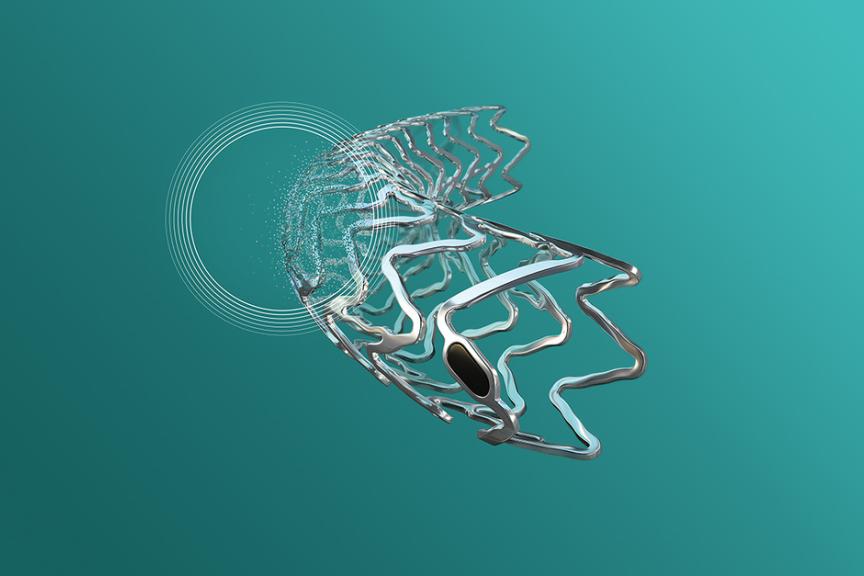
CE-approval for BIOTRONIK’s Next-Gen Metallic Bioresorbable Scaffold Freesolve Freesolve Resorbable Magnesium Scaffold Fully Resorbs at 12 Months and is Now Commercially Available in CE-Accepting Countries
BIOTRONIK announces the CE approval and launch of Freesolve™ Resorbable Magnesium Scaffold (RMS). This third generation RMS has been engineered to provide optimized vessel support, yet achieves magnesium resorption within 12 months.1 The new Freesolve RMS is a groundbreaking vascular advancement based on reliable clinical evidence. Recent BIOMAG-I trial data highlights an exceptional 99.3% magnesium strut degradation 12 months after implantation2, consistent performance, regardless of lesion characteristics, and restoration of vasomotion.3
"Having closely observed the evolution of resorbable magnesium scaffolds, the Freesolve RMS achieves a 99.3% resorption rate at 12 months and provides better scaffolding than its predecessors," said Prof. Michael Haude, Rheinland Klinikum, Neuss, Germany. "The enhanced properties of the Freesolve RMS can establish a true alternative to DES, benefitting from the advantages of a resorbable device while maintaining a reliable and predictable outcome with the long-term safety profile that has already been shown in many RMS trials with previous versions."
The Freesolve RMS is designed to enhance the lives of patients with de novo coronary artery lesions. It provides safety, improved deliverability, and optimal performance and vessel support during and after implantation. Furthermore, its versatility in sizes and lengths enables tailored treatments for diverse coronary anatomies. Featuring the proprietary and unique BIOmag® magnesium alloy, the Freesolve RMS stands in a class of its own given its natural compatibility with the human body.
The new resorbable magnesium scaffold introduces unique benefits:
- Improved deliverability: The Freesolve RMS incorporates the trusted Orsiro® Mission DES delivery system, thinner struts and new markers for increased radiopacity compared to previous generations.4
- Optimal vessel support: The proprietary BIOmag® magnesium alloy used to design the Freesolve RMS allows improved mechanical properties5 and prolonged radial support6,7.
- 12-months resorption*2: 99.3% of magnesium is resorbed at 12 months.
- Excellent safety and efficacy8,9: At 12-month follow-up, Freesolve RMS shows low Target Lesion Failure (2.6%) and clinically-driven Target Lesion Revascularization rate (2.6%), no myocardial infarction and no definite or probable scaffold thrombosis.
"We are very proud of being the leading company bringing this therapy revolution with exceptional clinical study results, Freesolve RMS, to the market, physicians and patients. BIOTRONIK RMS technology defines a new standard for resorbable scaffolds; having the benefit of metallic implants in the short-term and ultimately leaving nothing behind while using a highly biocompatible material - magnesium”, said Dr. Alexander Uhl, CEO at BIOTRONIK. “In my opinion, Freesolve RMS technology will become a new standard in every clinic to complement current therapy options ensuring optimal treatment for every patient.”
To grow the evidence base behind the Freesolve RMS further, BIOTRONIK will commence enrolment in the BIOMAG-II study in the second quarter of 2024, comparing the Freesolve RMS to contemporary DES. Building upon the findings of prior studies, this large, randomized controlled trial aims to encompass a comprehensive patient cohort exceeding 1,800 individuals across CE and APAC regions.
-END-
About Freesolve: A True BIOTRONIK Innovation
In 2016, BIOTRONIK´s Magmaris® Resorbable Magnesium Scaffold pioneered a new approach to resorbable technology as the first metallic resorbable scaffold in the CE area. Based on the excellent clinical outcomes of Magmaris RMS, BIOTRONIK continued to develop resorbable magnesium technology culminating in the Freesolve RMS – the next generation of RMS that incorporates several technical improvements to address the interventional cardiologists needs and for enable optimal patient outcomes.
For more information, please visit: Freesolve RMS
References:
* Markers are not resorbable
1.Seguchi M., BIOMAG-I: Twelve-months vessel healing profile following the novel resorbable magnesium scaffold implantation: an intravascular OCT analysis of the BIOMAG-I trial, presented at ESC 2023.
2. Seguchi M., “Twelve-months vessel healing profile following the novel resorbable magnesium scaffold implantation: an intravascular OCT analysis of the BIOMAG-I trial” presented at ESC August 2023.
3. Haude M. “A new resorbable magnesium scaffold for de novo coronary lesions (DREAMS 3G): 12-month results of the BIOMAG-I first-in-human study” presented at ESC August 2023.
4. BIOMAG-I Case, courtesy of M. Haude, Lukaskrankenhaus Neuss, Germany 2021
5. BIOTRONIK data on file compared to Magmaris® RMS, applicable for the scaffold diameter sizes 3.0 and 3.5 mm.
6. Masaru Seguchi et al. Preclinical evaluation of the degradation kinetics of third generation resorbable magnesium scaffolds. Eurointervention; 2023;18-online publish-ahead-of-print January 2023. DOI: 10.4244/EIJ-D-22-00718.
7. BIOTRONIK data on file.
8. Haude, M. et al, the Lancet eClinicalMedicine 2023;59: 101940.
9.Haude, M. et al, EuroIntervention 2023;19:1-1 published online May 2023.
Freesolve is not available for sale in the United States.
BIOMAG, Freesolve, Magmaris, Orsiro and Orsiro Mission are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
Download our Freesolve media fact sheet here.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.