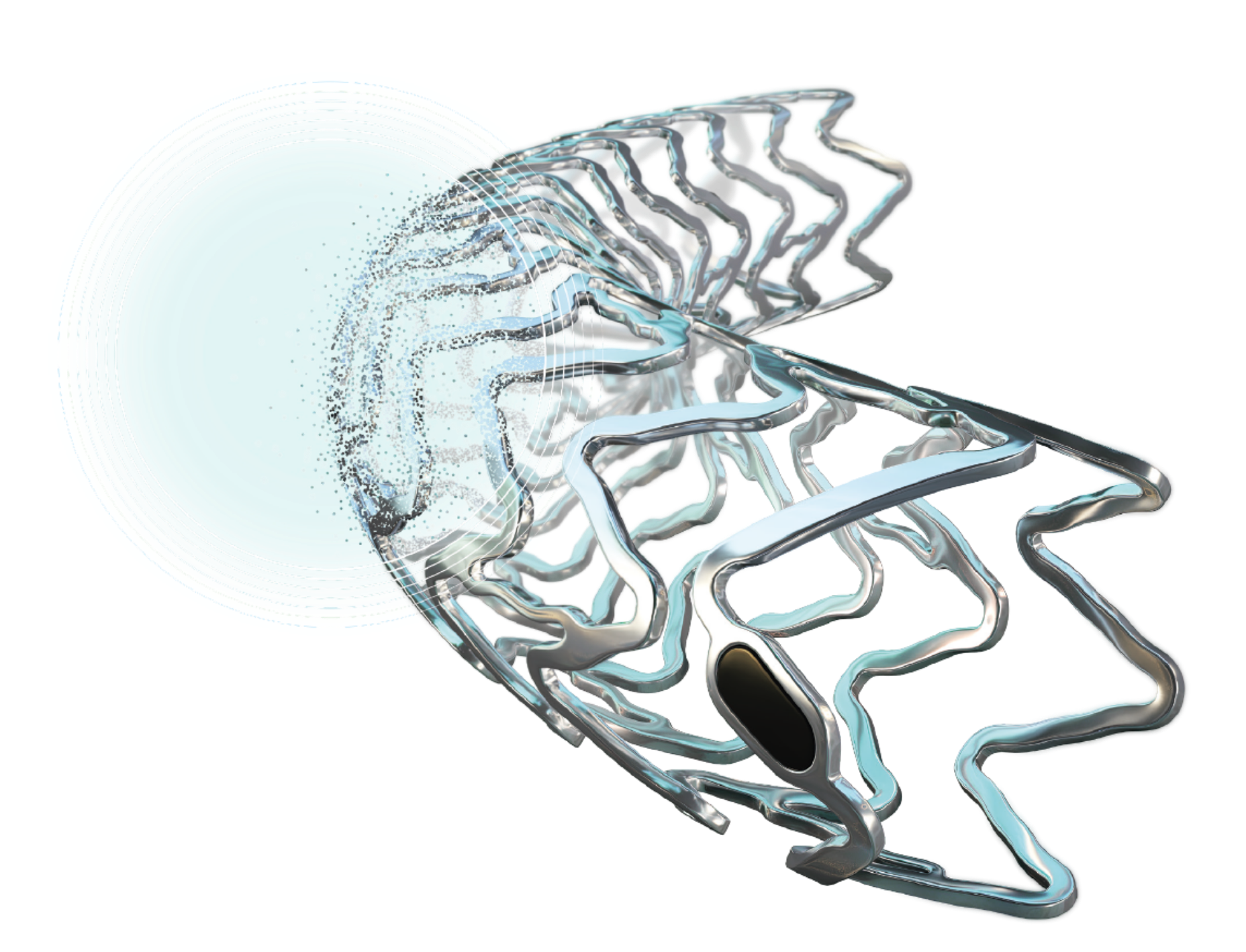
Resorbable Magnesium Scaffold (RMS)
Keen to learn more about Freesolve® RMS? Fill in the form below to download the product brochure.
Request more information and a BIOTRONIK representative will be happy to get in touch.
Get in touch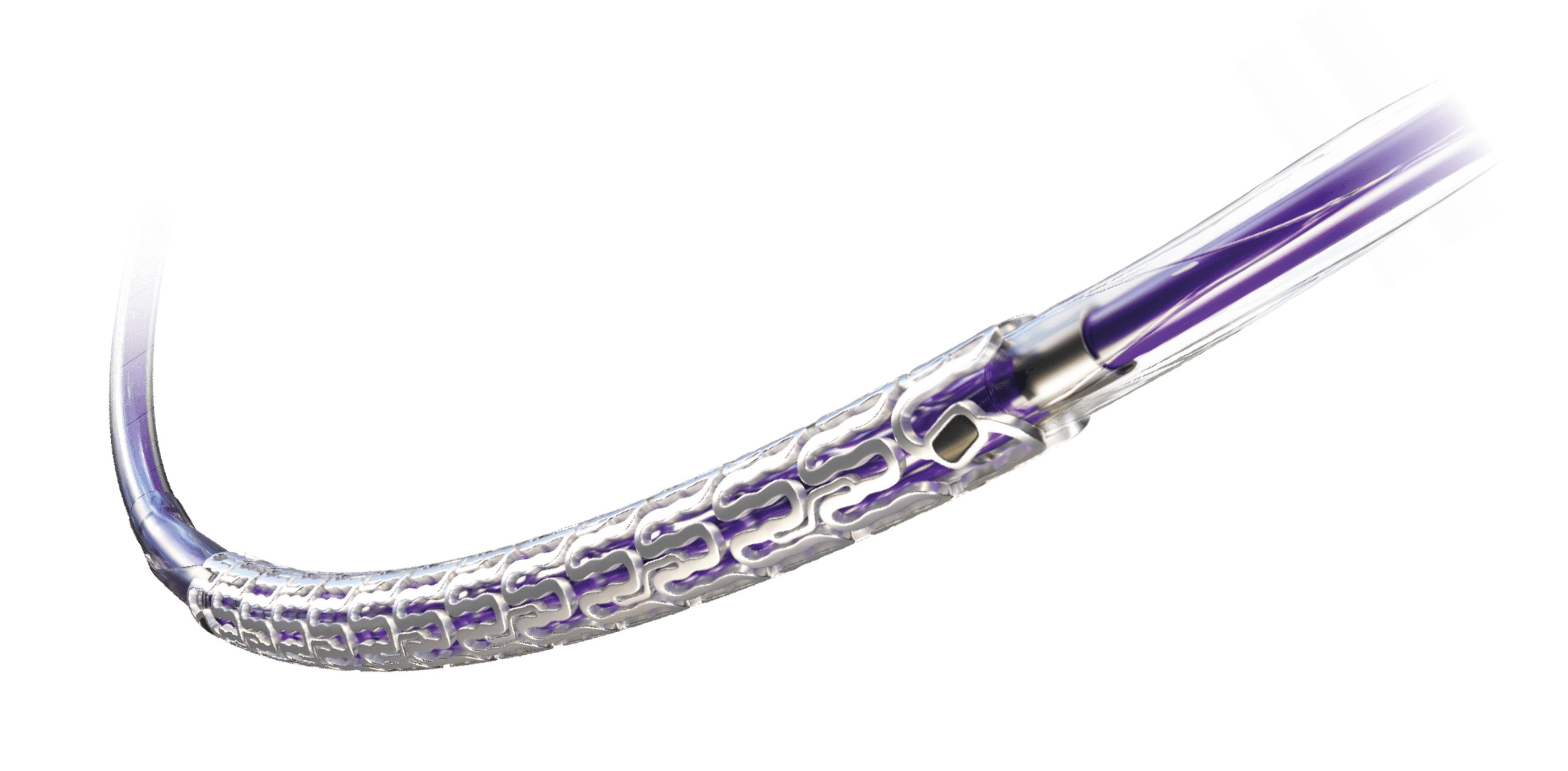





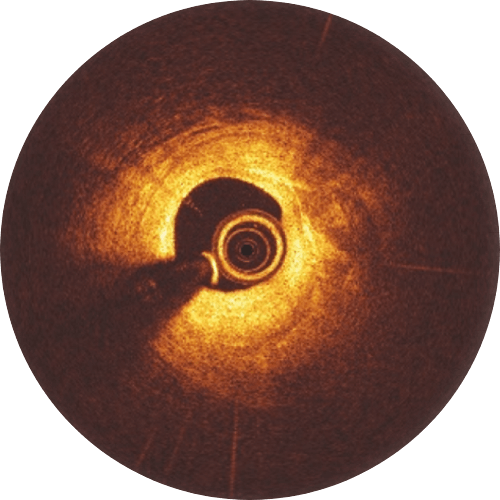
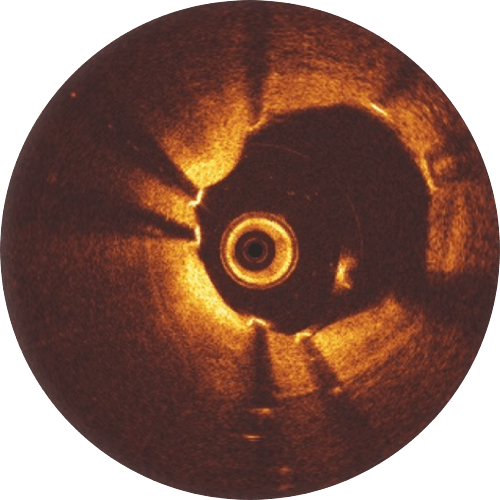
Experience shows that following 4 Ps for Freesolve RMS implantations may lead to better patient outcomes.
Appropriate patient selection is crucial to achieve procedural success. Freesolve is currently indicated for de novo lesions, with a reference vessel diameter and lesion length closely matching the available Freesolve sizes. Each individual patient should receive best clinical care and should benefit from BRS Technology.
If uncertain about the vessel diameter, use QCA, IVUS and/or OCT for quantitative lesion evaluation. The diameters available are 2.5, 3.0, 3.5 and 4.0 mm, do not implant into vessels <2.5 mm or >4.2 mm in diameter. Angiogram generally underestimates the diameter of the vessel by 0.25 mm.
Pre-dilatation with a non-compliant balloon with a 1:1 balloon-to-artery ratio is mandatory. The balloon should expand fully. The residual stenosis before the Freesolve implantation is recommended to be less than 20 %. If the pre-dilatation goal is not achieved, use other balloon technologies such as scoring balloons.
Post-dilatation with a non-compliant balloon 0.5 mm larger than the implanted scaffold expanded at high pressure (> 16 atm) is recommended. Please keep in mind that the Freesolve expansion limit is 0.6 mm beyond nominal scaffold size. During the learning phase, OCT is helpful to check for vessel and lumen dimensions, lesion length and struts’ malapposition.
A wealth of clinical evidence
*restricted access, sign-in required
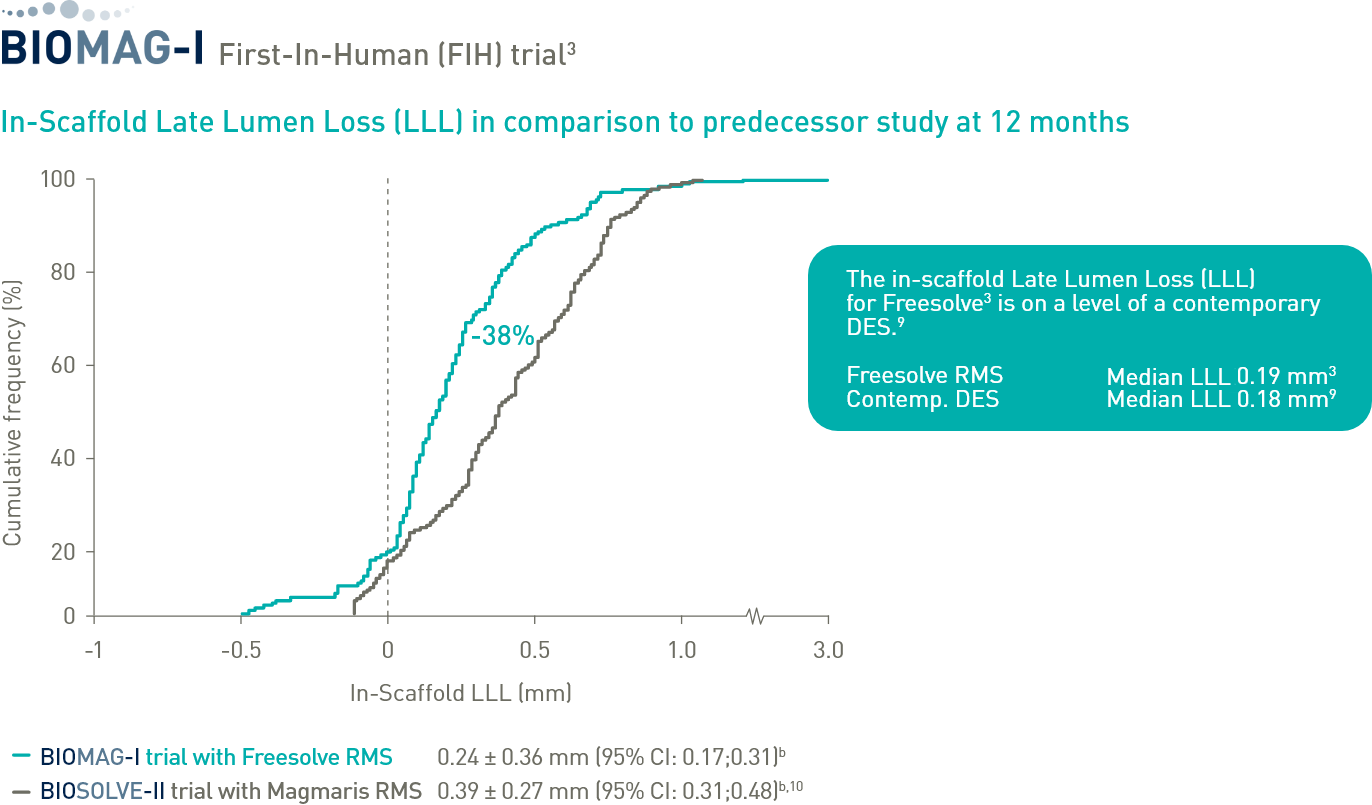
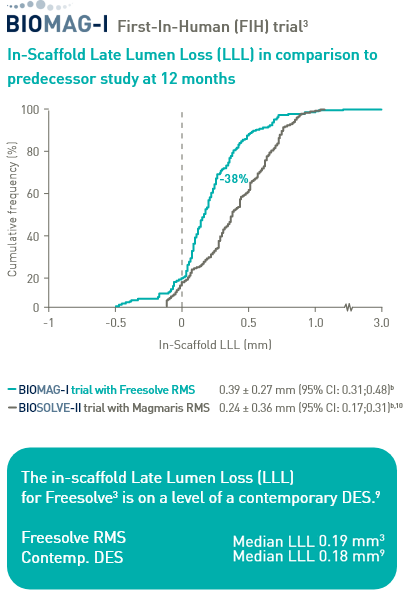
Prof. Michael Haude (Rheinklinikum, Germany) talks about Freesolve® and the BIOMAG-I First-In-Human trial outcomes and what impact this has for the future of scaffolds.
Prof. Michael Joner (German Heart Centre, Germany) explains the resorption process of the new generation RMS Freesolve® and how this scaffold can have thinner struts and a resorption time of 12 months with a prolonged scaffolding time.
Dr. Masaru Seguchi (German Heart Centre, Germany) explains the aim of the BIOMAG-I OCT analysis, what the findings are and what the data means for the future of cardiology.
Prof. Hector Garcia-Garcia (MedStar CV Research Network, Washington DC, US) talks about the AI-based OCT analysis from the BIOMAG-I FIH trial and how Freesolve® RMS triggers the thickening of the fibrous cap, contributing to plaque stabilisation.
Metallic Performance1-3. Fully Resorbable.a4
What would you choose?