
Biomonitoring in Patients with Preserved Left Ventricular Function after Diagnosed Myocardial Infarction
Jøns C et al, ACC 2022
First Outcome Trial on the Benefits of ICMs in Post-MI Patients

The BIO|GUARD-MI study is the first trial to investigate the impact of continuous arrhythmia monitoring with an implantable cardiac monitor (ICM) on clinical outcomes in post-myocardial infarction (MI) patients. Implantable cardiac monitors are also referred to as implantable loop recorders.
The study demonstrated that early treatment of high-risk, non-ST-segment elevation myocardial infarction (NSTEMI) patients guided by continuous arrhythmia monitoring with implantable loop recorders reduces major adverse cardiac events (MACE) by 31%.
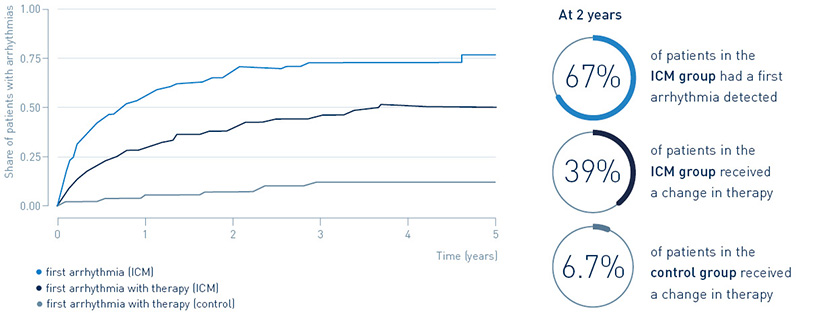
67% of patients with an implantable loop recorder had a first arrhythmia detected after two years, leading to guideline-recommended change in treatment in 39.4%. Only 6.7% of post-MI patients without an implantable loop recorder received a change in treatment.
Read more about the study design, key results, and clinical relevance below.
As presented at a Late-Breaking Clinical Trial session at the American College of Cardiology’s 71st Annual Scientific Session in Washington D.C. on April 4, 2022
Study Design
- Randomized, controlled, parallel-group, open, prospective, multi-center, international study
- To investigate whether the early diagnosis of cardiac arrhythmias, provided by the BIOMONITOR with Home Monitoring, and the consequent treatment of the patient will decrease the risk to experience a major adverse cardiovascular event (MACE) in patients after MI, with LVEF >35% and CHA2DS2-VASc score ≥4 (men) or ≥5 (women)
- 790 patients
Key Result 1
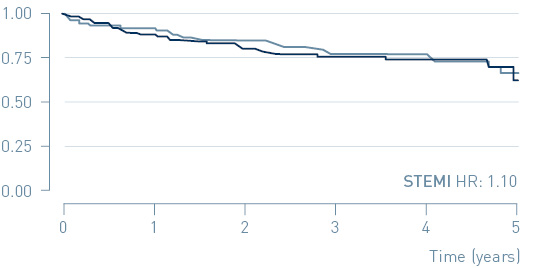
In the primary endpoint analysis of the total patient population, a trend towards MACE reduction in the implantable cardiac monitor (ICM) group was observed but did not reach statistical significance (HR=0.84, p=0.21).
A sub-group analysis shows a 31% reduction of MACE in patients with non-ST segment elevation myocardial infarction (NSTEMI).
Primary endpoint: Time to first MACE in NSTEMI and STEMI
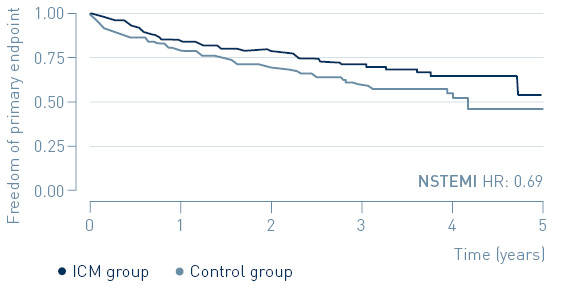
↓ 31%
MACE reduction with ICM in NSTEMI patients
Key Result 2
The benefit of ICM-guided treatment in NSTEMI patients appears to be connected to their higher risk for primary endpoint events.
Risk for primary endpoint in NSTEMI and STEMI
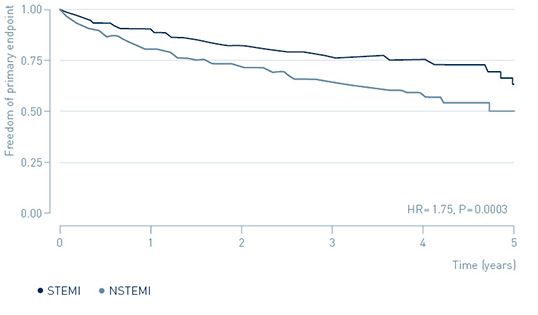
↑ 75%
increased risk of MACE in NSTEMI patients
Key Result 3
Continuous arrhythmia monitoring in post-MI patients identifies a large arrhythmia burden, and many of the arrhythmias require guideline recommended therapies.
Detection of arrhythmias and treatment
Clinical Relevance
- Arrhythmias are connected to poor outcomes after myocardial infarction.
- BIO|GUARD-MI is the first trial to investigate the impact of continuous arrhythmia monitoring with ICMs on clinical outcomes in post-MI patients.
- Early treatment of high-risk NSTEMI patients guided by continuous arrhythmia monitoring with BIOMONITOR and Home Monitoring may reduce major adverse cardiac events (MACE).
| Study Objective |
|
|---|---|
| Primary Endpoints |
|
| Sample Size |
|
| Clinical Sites |
|
| Main inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Flowchart | 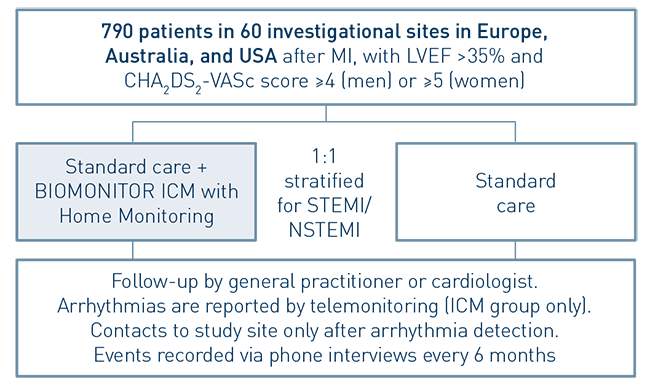 |
| Devices |
|
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Steering Committee |
|
