BIOSOLVE-I
NCT01168830
First-in man trial with DREAMS (Drug-Eluting Absorbable Magnesium Scaffold)
Conclusions
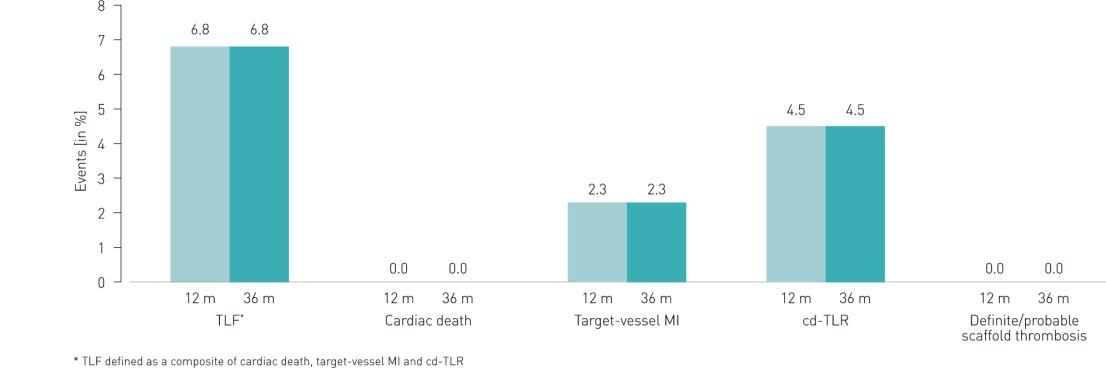
- Excellent long-term outcomes at 3 years with a low Target Lesion Failure (TLF) rate and no cardiac death or scaffold thrombosis
- No TLF events were observed after the first year
Study Design
Prospective, multi-center, First-In-Man trial testing DREAMS (Drug-Eluting Absorbable Magnesium Scaffold) in 46 patients with a total of 47 de novo lesions
Principal investigator: Prof. Michael Haude, Neuss, Germany
Primary Endpoint:
Target Lesion Failure (TLF) defined as a composite of cardiac death, target-vessel myocardial infarction (TV-MI) and clinically driven Target Lesion Revascularization (cd-TLR) at 6 and 12 months
Secondary Endpoints:
- Late Lumen Loss (LLL) at 6 and 12 months
- Scaffold thrombosis at 1, 24 and 36 months
- Cumulative rates of TLF at 1, 24 and 36 months
Clinical Results
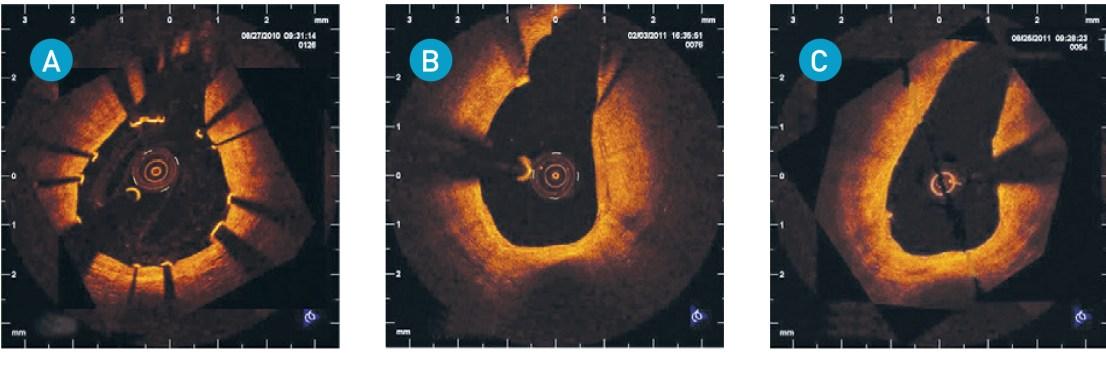
Representative optical coherence tomography after implantation of drug-eluting bioresorbable scaffolds (DREAMS) (A) at 6 months (B) and 12 months (C)
Immediately after implantation strut apposition to the vessel wall is good, with some struts covering the sidebranch. At 6 months remnants are mostly covered and former struts over the side branch are being resorbed. The change from a metallic stent-like appearance to remnants after magnesium resorption is shown.
Downloads/Links


Vascular Intervention
Clinical StudyFirst in man study of the DREAMS 2nd generation drug-eluting absorbable metal scaffold
Reference
Reference: Haude M et al. Safety and performance of the drug-eluting absorbable metal
scaffold (DREAMS) in patients with de novo coronary lesions: 12-month results of the
prospective, multi-centre, first-in-man BIOSOLVE-I trial. Lancet. 2013; 381: 836-44.
Disclaimer
Magmaris is not available in the US.
© BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.
