BIOSync CLS
Cardiac Pacing in Severe Recurrent Reflex Syncope and Tilt-Induced Asystole
Study Design
For more information about this study
- Randomized, placebo-controlled, prospective, double blinded, international, multicenter clinical trial
- Patients with severe reflex syncopies and previously implanted Eluna/Epyra 8 DR-T devices
- 1:1 randomized to DDD-CLS pacing vs. ODO mode (placebo; no pacing , dual chamber sensing only)
- Patients and Clinical Events Adjudication Committee were blinded to pacing mode
- Quarterly self-administered patient questionnaire to collect study endpoints
- Sequential study design with two interim analyses at 40% and 70% of the required endpoints
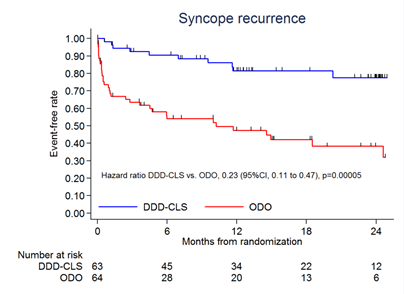
Key Result 1
CLS significantly reduced the syncope recurrence rate by 77% versus placebo
Figure 1: Survival free of syncope recurrence for CLS-paced patients versus placebo
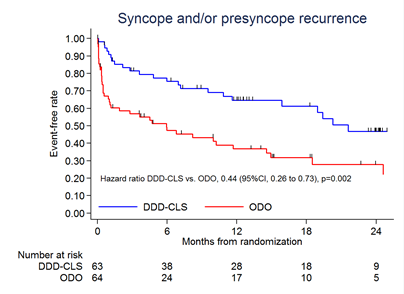
Key Result 2
CLS significantly reduced the combined rate of syncope and/or pre-syncope by 56% versus placebo
Clinical Relevance
- The BIOSync CLS study is the largest and best–designed randomized clinical trial to demonstrate the efficacy of cardiac pacing with CLS in patients suffering from severe recurrent reflex syncope
- So far, the usefulness of tilt-table testing to select patients with severe recurrent reflex syncope for cardiac pacing was controversial. The positive results of the BioSync CLS study indicate that asystolic response to tilt-table tests is a valuable selection criterion.
- By using a physiologic signal retrieved from the body’s own cardiovascular regulation, Closed Loop Stimulation is able to provide clinical benefit beyond physiological pacing therapy of chronotropic incompetent patients.
| Study Objective |
|
|---|---|
| Primary Endpoint |
|
| Major Secondary Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Main Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Study Flowchart | 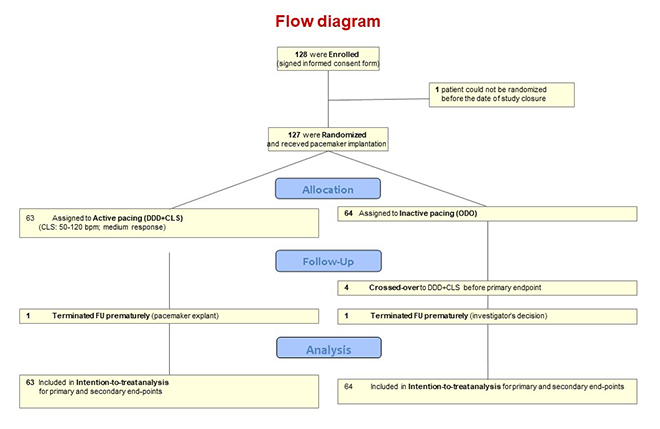 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
