RM-ALONE
Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial
GARCIA FJ ET AL., EUROPEAN HEART JOURNAL 2019
Study Design
- Prospective, randomized, multicenter clinical trial
- 445 pacemaker (PM) and ICD patients randomized 1:1 at 16 Spanish institutions
- Patient surveillance by Home Monitoring + remote device interrogations every 6 months (RM-ALONE protocol) vs. Home Monitoring + in-office evaluations every 6 months
- 24 months follow-up period
- To demonstrate the possibility to safely and efficiently dispense with face-to-face follow-up visits by the RM-ALONE protocol for both pacemaker – and ICD-bearing patients
Key Result 1
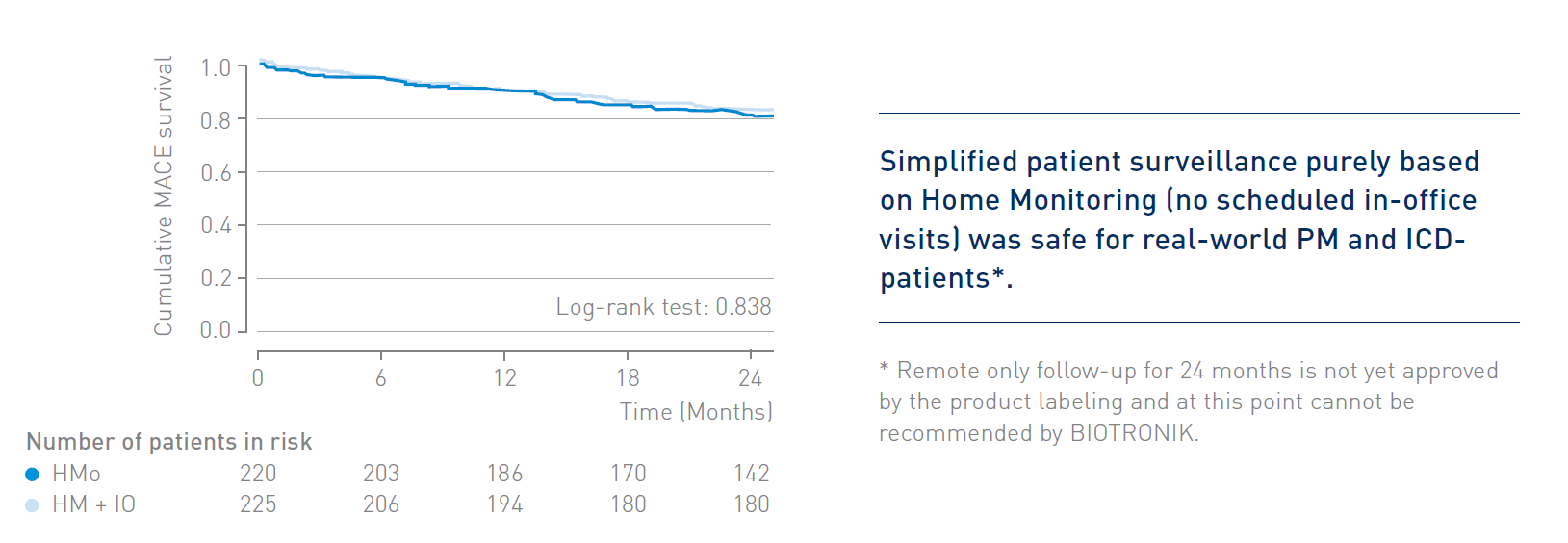
The RM-ALONE protocol demonstrated non-inferiority in terms of safety in comparison to continuous remote monitoring associated with on-site visits every 6 months for the overall population of PM- and ICD patients.
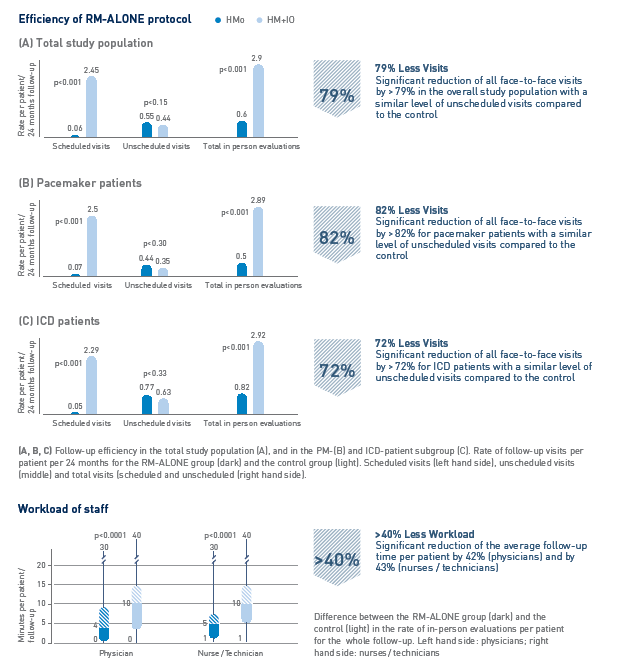
Key Result 2
Patient surveillance exclusively based on Home Monitoring (RM-ALONE Protocol) reduces number of follow-up visits and clinical workload
Clinical Relevance
- RM-ALONE is the first randomized trial surveilling PM and ICD patients with a simplified uniform follow-up pattern and using remote monitoring only as a gold standard in both groups.
- Before RM-ALONE, the main concern about extending the time between in-office follow-ups was that safety may be compromised. The RM-ALONE results indicate that surveillance of a real-world PM- and ICD- patient population exclusively based on remote monitoring is safe.
- CIED follow-up is the most frequent activity reported by cardiac electrophysiologists. By following the RM-ALONE follow-up protocol, the clinical workload resulting from follow-up activities can be significantly reduced for pacemaker and ICD patients.
| Study Objective |
|
|---|---|
| Primary Endpoint |
|
| Major Secondary Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Main Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Follow-Up |
|
| Study Duration |
|
| Principal Investigators |
|