BIOFLOW-II
NCT01356888
Study of the Orsiro Drug Eluting Stent System
Conclusion
- Target Lesion Failure (TLF) comparable to Xience Prime* although separating over time in favor of Orsiro out to 48 months
- Absence of definite or probable stent thrombosis also in high risk populations such as diabetic and small vessel subgroup out to 48 months
- The results of this prospective, randomized study confirms the safety and efficacy of Orsiro
Study Design
A prospective, multi-center, randomized, controlled trial comparing the Orsiro DES to Xience Prime
Coordinating clinical investigators:
Prof. Stephan Windecker, Bern, Switzerland and Dr. Thierry Lefèvre, Massy, France
Primary endpoint:
In-Stent Late Lumen Loss (LLL) at 9 months
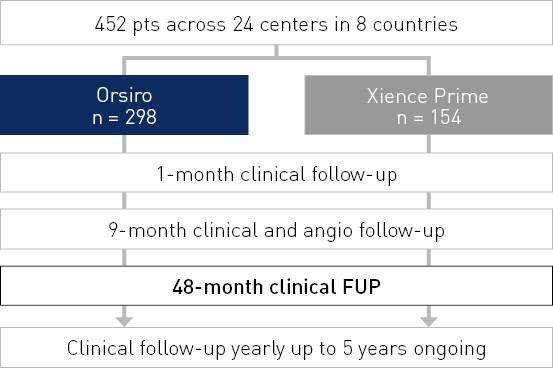
Results at 48 months
TLF rate – All subjects
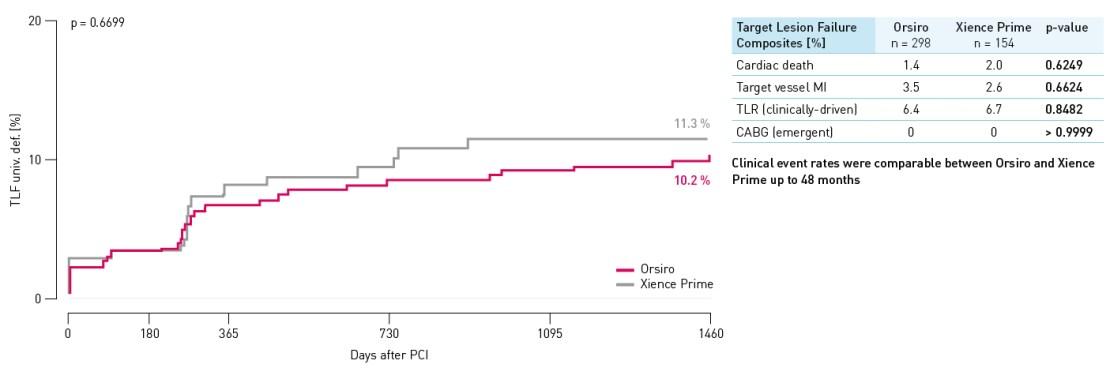
TLF rate – Small vessel population
TLF defined as composite of cardiac death, target vessel Q-wave or non Q-wave Myocardial Infarction (MI), Coronary Artery Bypass Grafting (CABG) and clinically-driven TLR.
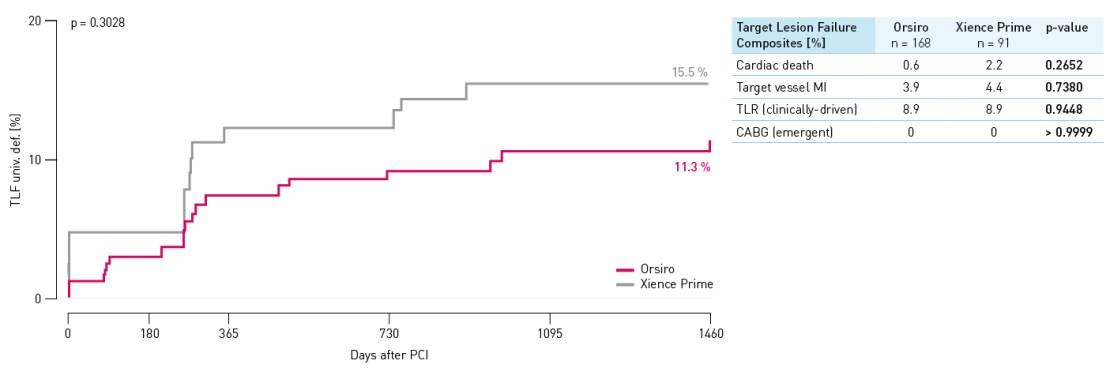
All subject stent thrombosis results at 48 months
No definite and no probable stent thrombosis occurred in either arm through 48 months


Disclaimer
© BIOTRONIK AG – All rights reserved. Specifications are subject to modification, revision and improvement.
*Xience and Xience Prime are registered trademarks of Abbott Cardiovascular Systems.Reference: Ton Slagboom on behalf of the BIOFLOW-II Investigators, Poster, euroPCR 2017
