TASC D Registry
Superficial femoral artery (SFA)
Twelve months effectiveness analysis of the Pulsar-18 self-expanding nitinol stent in patients with critical limb ischemia1
Conclusion
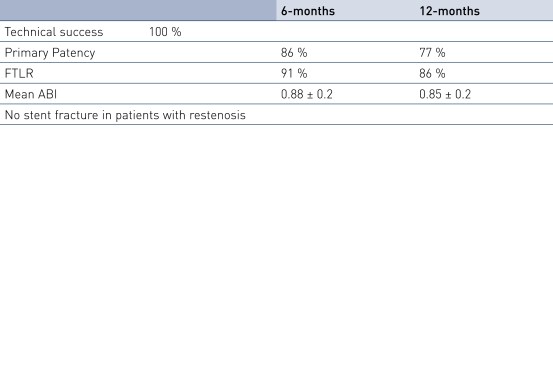
- SFA TASC D lesions can be treated with the Pulsar-18 stent with 100% procedural success in critical limb ischemia (CLI) patients.
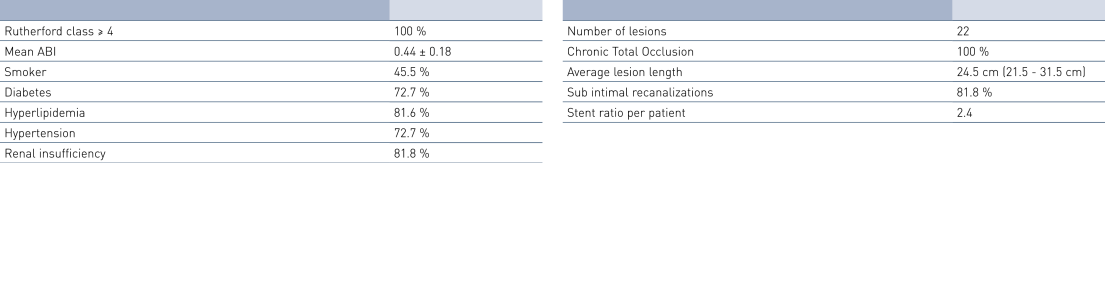
- The average lesion length of 24.5 cm is much longer than in other published data, while primary patency (PP) and Freedom from Target Lesion Revascularization (FTLR) rates are similar to this published data.
- In these very long and chronic total occluded lesions, the Pulsar-18 stents provide sufficient radial force, as demonstrated by 77% PP at 12 months.
Study Design
- Single-center, prospective registry
- Number of patients (n): 22
- Primary investigator: Dr. Michael Lichtenberg, Klinikum Arnsberg, Germany
- Follow-up at 6 and 12 months
- Endpoints: primary patency (PP)2 2 at 12 months and freedom from Target Lesion Revascularization (FTLR)
Patient Demographics and Lesion Characteristics
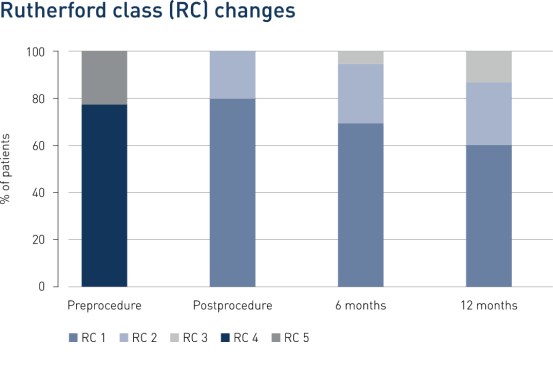
Results
Downloads

Vascular Intervention
Self-expanding StentOne-handed stent release for accurate stent deployment
Vascular Intervention
Self-expanding StentTri-axial shaft for a stable delivery system during stent deployment
1 Lichtenberg M, Stahlhoff W, Boese D. J. Cardiovasc Surg. 2013; 54: 433-9.
2 = Peak Systolic Velocity Ratio < 2.5 m/s duplex ultrasound
Not available for sale in the USA. CAUTION - Investigational Device.
Limited by United States Law to Investigational Use.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
