Rivacor 7 HF-T QP/HF-T
More Life. Made Simpler
Simplified and optimized CRT for up to 9 years[1]
Heart failure is tough to live with and can be complex and time-consuming to treat and manage. It’s these real life challenges that Rivacor 7 CRT-D systems are engineered to deal with. These new and smaller systems simplify treatment and therapy for up to 9 years1, and when used with BIOTRONIK Home Monitoring technology, they have been clinically proven to help prolong life further[30].

BIOshape. Ultraslim 10mm CRT-D
Rivacor CRT-Ds are small and slim – only 10mm from front to back – with a smooth, elliptical, and body-friendly BIOshape[1].
- Rivacor CRT-Ds are the smallest, slimmest devices that are approved for 3T full-body MRI scans[2].
BIOshape eases implantation and reduces skin pressure[3],[4]. It helps to lower the risk of skin erosion while increasing patient comfort[5].
- The smooth, elliptical form facilitates insertion procedures. On average, four out of five implanting physicians rated ‘ease of device insertion’ and ‘securing in the pocket’ as ‘better’ or even ‘much better’ than with preceding models[6].
- Thinner design and rounded edges reduce skin pressure[7] and help to lower the risk of skin erosion.
- Over 90% of implanting physicians rated ‘patient comfort’ as ’better’ or even ‘much better’ in comparison with previous models[8].
9 years longevity. 6 years warranty
Rivacor CRT-Ds have an extended battery life of 9 years[9], supported by a full 6-year warranty.
- Rivacor CRT-Ds live up to 9 years and are the smallest, slimmest 3T FBS devices that live that long[10].
- Extended warranty reduces financial risk for implanting clinics, as fewer devices for replacement procedures will need to be purchased.
This can lead to fewer box replacements and fewer procedures for patients, reducing risks, complications and costs.
- For CRT patients, who already face a higher risk burden, increased device longevity can reduce the number of replacements. These patients are exposed to higher risk of complication than single- or dual-chamber ICD patients[11].
- Saving a replacement can lead to lower risk and less distress for CRT patients.
- Fewer replacements means reduced procedure costs. At the same time, complication-related costs can be avoided by reducing risks resulting from replacements.
Full 3T MRI. Autodetected
All Rivacor devices feature ProMRI, which gives full and uncompromised access to high-resolution MRI, with full-body scanning and no exclusion zone.
- 3 Tesla MRI offers the best image quality, required for example for neuroimaging. This standard significantly reduces scanning time and increases hospital efficiency.
- Full-body MRI means no exclusion zone and hence allows for the assessment of the cardiac anatomy, function and mass[12].
MRI AutoDetect senses MRI environments and self-adjusts, suspending tachycardia therapy for the duration of the scan only. This can be activated with a single visit to the physician and has a 14-day window for planning.
- MRI AutoDetect is a sensor-based technology that ensures optimal therapy and simplifies workflows in MRI.
- Upon activation, MRI AutoDetect automatically recognises an MR environment for up to 14 days. It provides the shortest time in MRI mode possible through automatically switching in and out of MRI mode. This minimizes tachycardia therapy suspension to the duration of the actual scan.
- MRI AutoDetect can be active for up to 14 days, allowing for multiple scans and scheduling flexibility regarding time and location. It provides a complete Home Monitoring report after the scan, meaning only one in-office visit is required[13].


>60% mortality reduction
Heart failure is a serious and progressive condition that puts CRT patients at risk in multiple ways. For these patients, AF is a common cause for loss of CRT pacing, while hospitalisation for decompensation is a frequent concern. Therefore, clinical guidelines recommend close multiparameter monitoring, because time is of the essence.
When CRT-Ds are used with BIOTRONIK Home Monitoring, life can be further prolonged with a clinically-proven > 60% reduction in all-cause mortality[14].
- Early detection of clinically relevant events through BIOTRONIK Home Monitoring enables early intervention[15].
- The IN-TIME trial shows that all-cause mortality can be reduced by >60%[16]. This outcome can be achieved by following the IN-TIME protocol, based on daily transmissions.
- BIOTRONIK Home Monitoring can be easily programmed to IN-TIME settings, enabling 28 alerts with the click of a button. This avoids the hassle of manually adjusting the alert settings to trigger ‘IN-TIME events’.
Home Monitoring works via a smartphone-size, ‘plug in and go’ CardioMessenger Smart mobile unit, which is quick and easy to use[17] and transmits data without any patient involvement.
- BIOTRONIK's 'plug in and go' solution allows for user-friendly system initialization. Data transmission is completely automatic, not requiring any active patient involvement[18], while the smartphone-sized CardioMessenger Smart transmitter makes BIOTRONIK Home Monitoring a mobile system.
- Daily, system-based transmission checks allow for industry-leading transmission success rates of 85%[19],[20].
- 99% of patients agree that BIOTRONIK Home Monitoring is easy to use, while 98% express their complete satisfaction with the system[21].
QuickCheck gives access to patient data typically within 3 to 4 minutes[22] for more efficient treatment and greater peace of mind for patients.
- QuickCheck provides fast access to patient data. Upon the physician’s request in the Home Monitoring Service Center, data typically becomes available within 3 to 4 minutes[23].
- Patient device data can be transmitted without patient interaction.
- This allows clinicians to be responsive to patients' needs remotely, while patient satisfaction may be positively influenced by the knowledge that the physician has access to current cardiac device data.
- Immediate clarification and patient reassurance might be complemented by a scheduled visit (for instance, on the next day), reducing the need for spontaneous hospital visits.
Continuous CRT optimization
With a rate of 30-40% non-response, CRT optimisation is a frequent need in CRT. Yet standard optimisation methods for CRT are time and resource-consuming. Suboptimal AV timing is considered the main cause for CRT non-response. Furthermore, fusion of intrinsic RV conduction with LV pacing has been found to have clinical advantages.
CRT AutoAdapt provides continuous CRT adaptation, automatically adjusting to changes in patient condition every minute.
- To fit each patient's individual and dynamic pacing needs, CRT AutoAdapt continuously adjusts the AV delay and pacing chamber selection, taking the patient's individual A-RV and A-LV conduction patterns into account.
- In patients with sinus rhythm and normal AV conduction, LV-only pacing with appropriate AV delay can result in superior ventricular function[24].
- In left bundle branch block (LBBB) patients, switching to LV-only pacing and adjusting the AV delay can provide optimal interventricular fusion between the activation fronts from the intrinsic RV conduction and the LV pacing lead[25]. For every patient, CRT AutoAdapt continuously evaluates whether an LBBB pattern is present by comparing A-RV and A-LV conduction time. In case of LBBB, CRT AutoAdapt adjusts the pacing chamber selection accordingly.
- By reducing pacing to LV-only, battery life can reach up to 10.8 years, even with daily remote data transmission[26].
- While the algorithm is transparent and easy to follow, additional programming options are available to further adjust for individual patients, if needed.

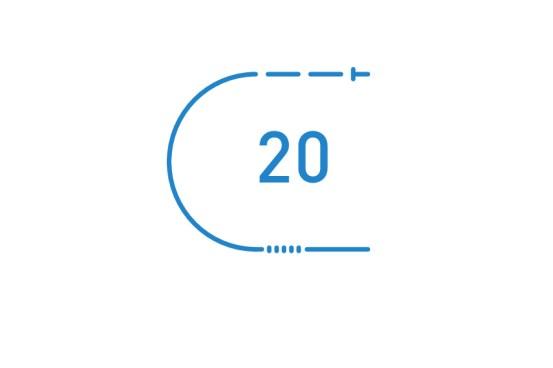
Auto LV VectorOpt helps evaluate clinically relevant pacing parameters, enabling automatic threshold measurements in minutes[27] and rapid direct programming with a choice of 20 vectors.
- Rivacor CRT-Ds offer an industry-leading choice of 20 LV pacing polarities[28].
- Auto LV VectorOpt provides automatic threshold measurements across 20 vectors in less than 2.5 minutes[29].
- The tool gives instant access to parameters that indicate the optimal vector choice such as pacing and PNS thresholds, RV-LV conduction times, usable range, impedance and impact on remaining service time.
- With Auto LV VectorOpt, it is possible to preselect cathodes and vectors. Users can sort test results by preference, and program the device directly from the test page.
1 Contoured housing; Acticor/Rivacor CRT-D QP: 60x75x10mm; 35ccm; CRT-D: 60x71.5x10mm; 33ccm
2 as part of an MR conditional system
3 Post-Market observation; interim-analysis, December 21, 2018. Data on file.
4 Device shape analysis, February 2019. Data on file.
5 Post-Market observation; interim-analysis, December 21, 2018. Data on file.
6 Post-Market observation; interim-analysis, December 21, 2018. Data on file.
7 Device shape analysis, February 2019. Data on file.
8 Post-Market observation; interim-analysis, December 21, 2018. Data on file.
9 Acticor/Rivacor HF-T QP, 9.3 years @ 60 ppm; RA 15%, RV/LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year. Data on file.
10 Acticor/Rivacor HF-T QP: 9.3 years @ 60 ppm; RA 15%, RV/LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year: Data on file vs. Medtronic 3T FBS; CLARIA (AMPLIA, COMPIA) MRI QUAD CRT-D SureScan: 6.8 years; Competitor device manuals as of Nov. 2018
11 Lee DS et al. Evaluation of Early Complications Related to De Novo Cardioverter Defibrillator Implantation. Insights From The Ontario ICD database. J Am Col Cardiol 2010; 55:774-82
12 Fact file: Cardiac Imaging with MRI, CT and Nuclear Techniques, British Heart Foundation. January 2010.
13 When patients are monitored by BIOTRONIK Home Monitoring. See ProMRI® manual for all details.
14 Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590
15 Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up: The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010;122: 325 – 332.
16 Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590; (3.4% in the Home Monitoring Group vs. 8.7% in the control group)
17 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
18 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
19 Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590
20 Vs. 55% - Crossley G H, Boyle A, Vitense H, et al. The CONNEXT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial. JACC. 2011; 57(10):1181-1189 [for bar chart comparison only]
21 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
22 Performance analysis. Data on file, 2018
23 Performance analysis. Data on file, 2018
24 Martin DO et al. Heart Rhythm 2012;9(11):1807-1814
25 van Gelder BM, Bracke FA, Meijer A, Pijls NH. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol 2005;46:2305–2310.
26 Acticor/Rivacor HF-T QP, 10.8 years @ 60 ppm; RA 15%, RV 0%, LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year. Data on file.
27 Post-Market observation; interim-analysis, December 21, 2018. Data on file.
28 QP models only
29 Post-Market observation; interim-analysis, December 21, 2018. Data on file.
30 Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590; (3.4% in the Home Monitoring Group vs. 8.7% in the control group).
