BIOLUX P-I
NCT01221610
Clinical trial to assess the safety and performance of the coated Passeo-18 Lux paclitaxel-releasing PTA balloon catheter versus the uncoated Passeo-18 balloon catheter for treatment of stenosis of the femoropopliteal arteries
Conclusion
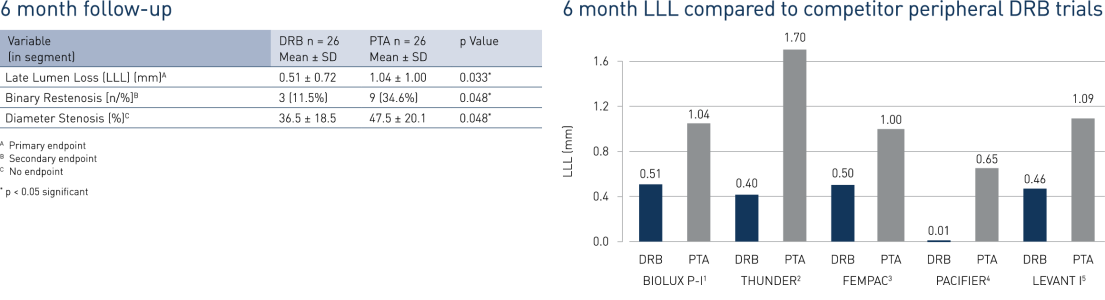
- In BIOLUX P-I, the Passeo-18 Lux drug-coated balloon demonstrated a significant reduction in late lumen loss (LLL) and binary restenosis at 6 months compared to the percutaneous transluminous angiography (PTA) control balloon.
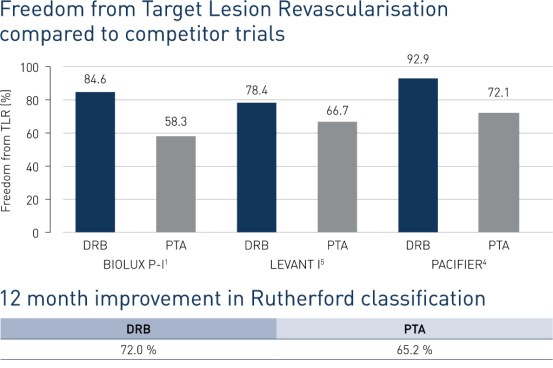
- At 12 months, freedom from Target Lesion Revscularisation (FTLR) was achieved in 84.6% of DRB patients and 58.3% of PTA patients.
- In addition, patients receiving treatment with Passeo-18 Lux showed greater improvement in Rutherford class compared to baseline (72%) vs. those receiving treatment with PTA (65.2%).
- At 12 months, Passeo-18 Lux demonstrated significantly better clinical performance compared to the control PTA balloon, which was in line with data from similar, competitor drug-coated balloon (DCB) randomised clinical trials.
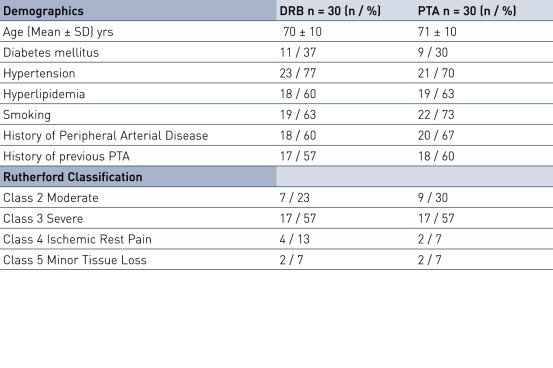
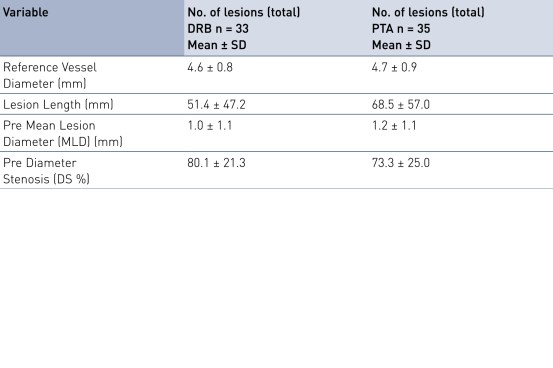
Baseline Characteristics and Lesion Characteristics
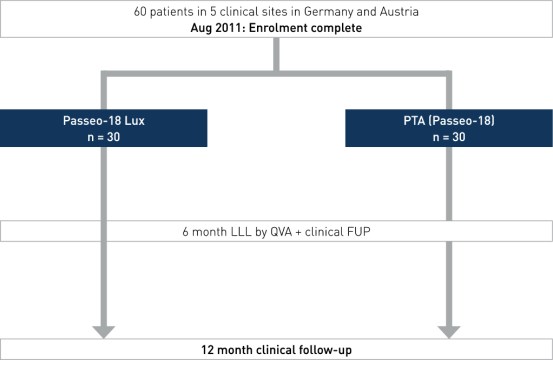
Study Design
Prospective, multi-center, 1:1 randomized, controlled trial enrolling 60 patients at five clinical sites in Germany and Austria.
- Number of patients (n): 60
- Clinical Sites: 5 sites in Germany and Austria
- Principal investigator: Dr. Dierk Scheinert, Leipzig, Germany
- Primary endpoint: 6-month LLL in target lesion measured by quantitative vascular angiography (QVA) by an independent corelab
- Secondary endpoints: 6-month binary restenosis, 6- and 12-month TLR, 6- and 12-month change in mean ankle-brachial index (ABI) and Rutherford class, major adverse event (MAE) at 6 and 12 months (procedure or device-related death or amputation, TL thrombosis, clinically driven TLR)
6-Month Follow-up
12-Month Results (Kaplan-Meier Estimates)
Downloads
Vascular Intervention
Drug-Coated BalloonClinically proven to reduce restenosis and the need for reinterventions

Vascular Intervention
PTA BalloonHighly pushable coaxial shaft design for most distal lesion access
Sources:
1 Scheinert et al. BIOLUX P-I. Presented at LINC 2013.
2 Thunder Tepe et al. N Engl J Med. 2008; 14; 358(7): 689-99.
3 Fempac Werk et al: Circulation. 2008; 118: 1358-1365.
4 Pacifier Werk et al. Circ Cardiovasc Interv. 2012; 5(6): 831-40.
5 Levant I: Scheinert et al. Presented at TCT 2010.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
