DELUX Registry
NCT01081366
Drug-Releasing Pantera Lux PTCA Balloon Catheter Registry
Conclusion
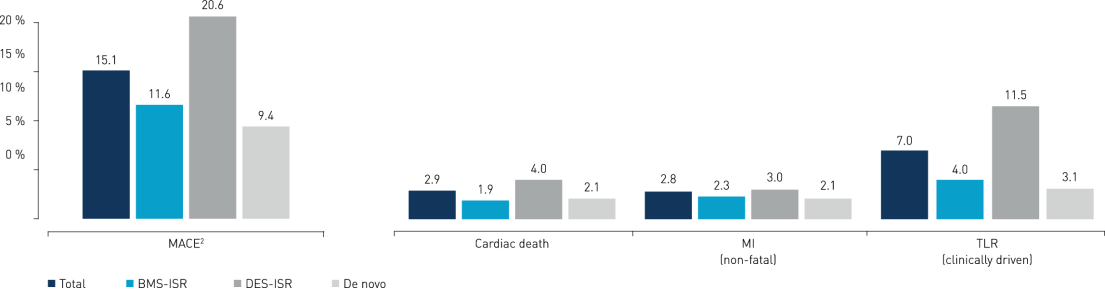
- Treatment with the Pantera Lux paclitaxel-coated balloon showed good 12-month outcomes in an international real-world setting in a predominantly difficult in-stent restenosis (ISR) population.
- Efficacy and safety are demonstrated by low revascularization, myocardial infarction (MI) and cardiac death rates and confirm previous clinical results of this device using butyryl-tri-hexyl citrate (BTHC) as an inert excipient.
- Results are favorable both in the overall population and in the de novo lesion subgroup.
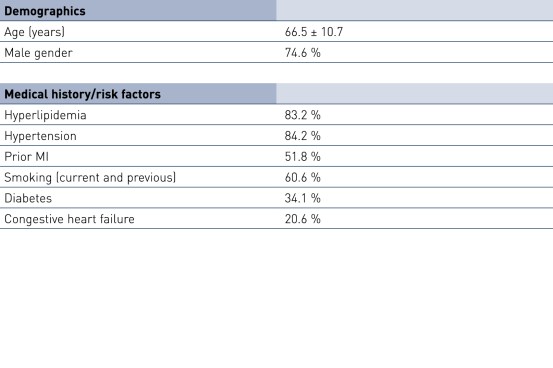
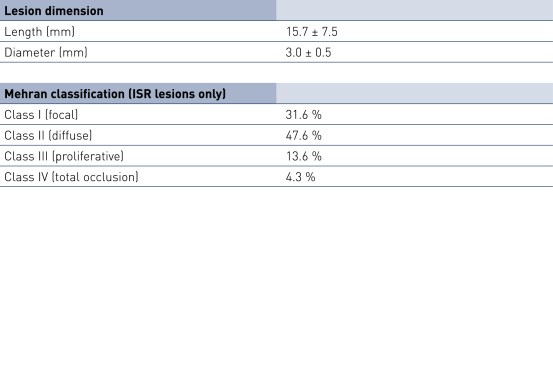
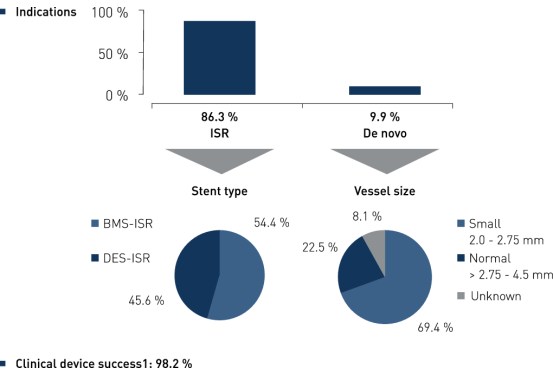
Baseline Characteristics and ISR Distribution by Stent Type
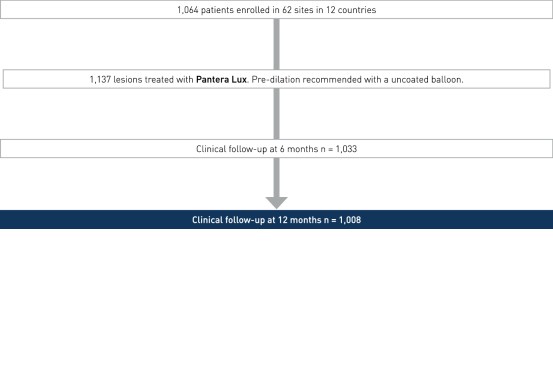
Study Design
- Prospective, multi-center international registry
- Number of patients (n): 1,064
- Principal investigator: Dr. Ralph Tölg, Herzzentrum Segeberger Kliniken, Bad Segeberg, Germany
- Clinical Sites: 62 sites in 12 countries
12-Month Results
Procedural Results
Downloads
Vascular Intervention
Paclitaxel-Releasing BalloonClinically proven solution in both in-stent restenotic and de novo lesions

Vascular Intervention
Clinical StudyOptimizing treatment of drug-eluting stent in-stent restenosis 4
1 Defined as successful deployment of device and < 50% residual stenosis of target lesion by visual estimation
2 Hierarchical MACE, composite of all death, non-fatal MI and clinically driven target vessel revascularization (TVR), adjudicated by clinical events committee
Source:
Tölg R. EuroIntervention. 2014; 10(5): 591-9.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
