More than 3,900 patients treated in clinical studies1-12,15-16
Pantera Lux DCB has proven efficacy and safety in multiple clinical trials investigating coronary drug-coated balloons for various implant-free treatment options.1-10
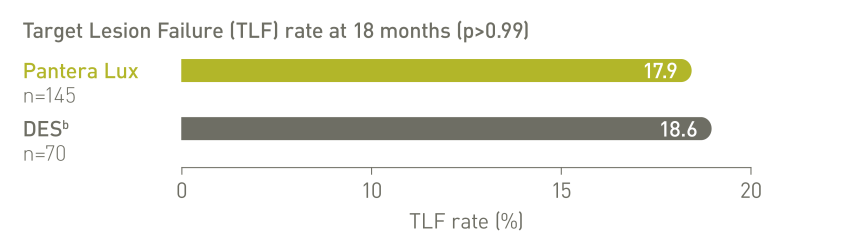
BIOLUX RCT (n=229)⁴
DCB is confirmed as a viable treatment option for ISR with the advantage of avoiding an additional stent layer.
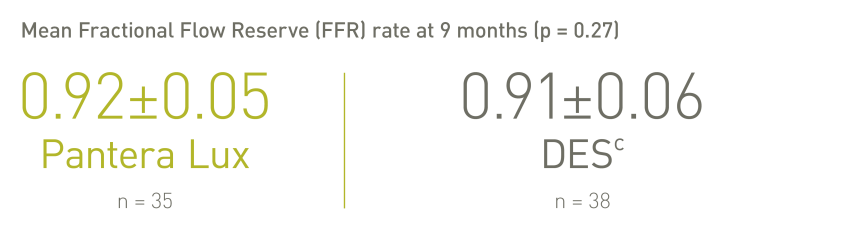
REVELATION (n=120)⁸
The treatment with Pantera Lux DCB may represent a valuable alternative strategy in selected STEMI patients undergoing primary PCI.