TRUST
Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up
Varma, Circulation 2012
Study Design
- Multi-center, prospective, randomized study
- Assesses that BIOTRONIK Home Monitoring can safely reduce in-office follow-ups
- 1,450 patients enrolled at 102 centers in USA
Key Result 1
TRUST demonstrated equal safety event rate in both groups. BIOTRONIK Home Monitoring delivered a reduction of 45% of in-office follow-up visits.
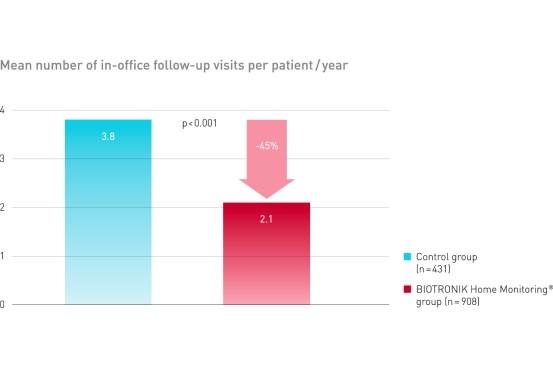
Key Result 2
BIOTRONIK Home Monitoring delivered a significant gain in early detection of clinically relevant symptomatic and asymptomatic events
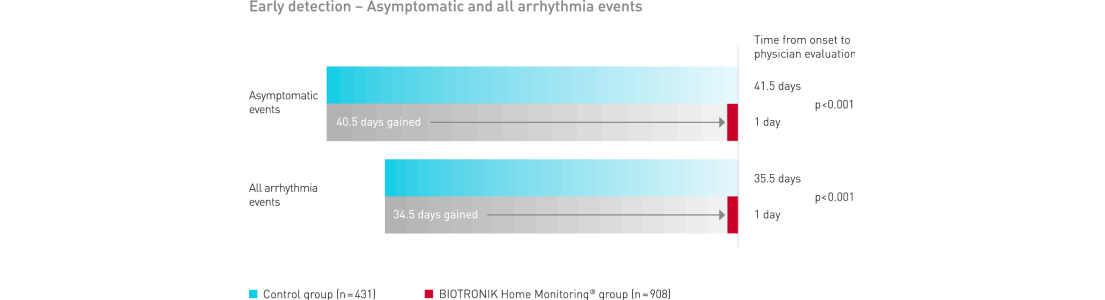
Key Result 3
BIOTRONIK Home Monitoring significantly reduced the time to evaluation of clinically relevant tachyarrhythmia events
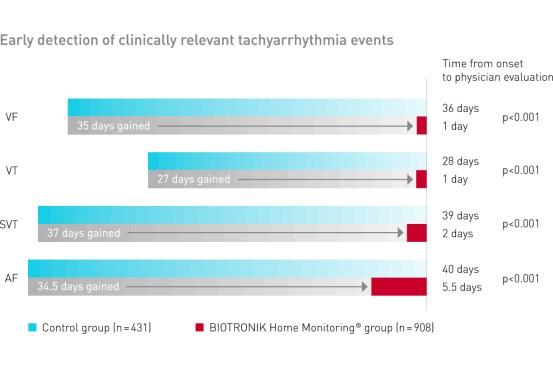
Clinical Relevance
- The TRUST trial is the first and largest clinical study of its kind. It has clinically proven the safety and effectiveness of BIOTRONIK Home Monitoring
- Results unequivocally establish BIOTRONIK Home Monitoring as a highly effective method of follow-up. Patients' safety is enhanced by enabling prompt medical care if problems occur in either their clinical condition or in their devices
| Study Objective |
|
|---|---|
| 1 Endpoint |
|
| 2 Endpoint |
|
| Clinical Sites |
|
| Sample Size |
|
| Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Devices |
|
| Study Flowchart |
 |
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
Downloads
Related Products

Tachycardia Therapy
BIOTRONIK offers an extensive product portfolio in the area of tachycardia therapy.

Cardiac Remote Monitoring
BIOTRONIK offers Home Monitoring for its complete product portfolio.
