4EVER
NCT01413139
Physician-initiated trial investigating the safety of the full 4F endovascular treatment approach of infra-inguinal arterial stenotic disease.1
Conclusion
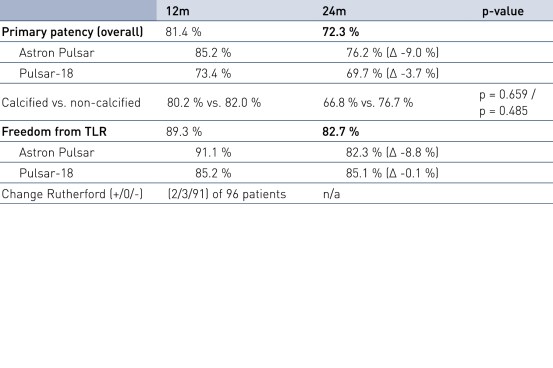
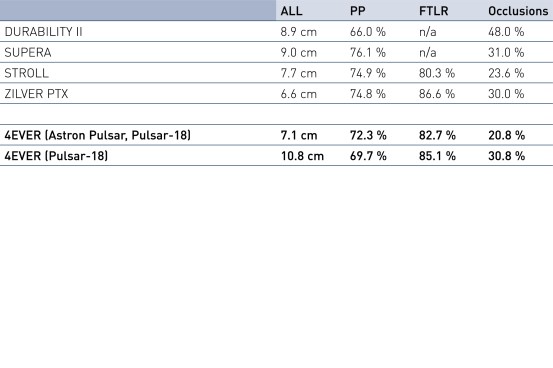
- Pulsar stents are safe and effective for treating SFA disease with excellent performance and clinical outcomes: Primary patency (PP) and Freedom from Target Lesion Revascularization (FTLR) in line with other documented bare metal/passive coated stents in lesions with similar characteristics; PP in line with Zilver PTX (Drug-eluting stent) even though longer average lesion length in 4EVER study; Sufficient chronic outward force and compression resistance demonstrated by the favorable 24-month PP, even in calcified lesions and occlusions.
- Astron Pulsar and Pulsar stents are safe and effective for treating SFA disease.
- Clinical outcomes in line with other documented studies including Zilver PTX.
- Sufficient radial force and compression resistance.
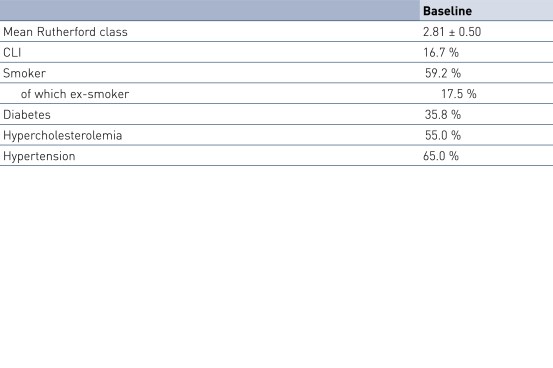
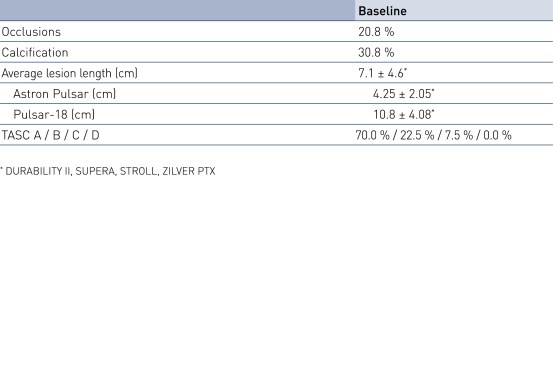
Patient Demographics and Lesion Characteristics
Study Design
- Number of patients (n): 120
- Primary Investigator: Dr. M Bosiers, A.Z. Sint-Blasius, Dendermonde, Belgium
- Primary endpoint: Primary Patency (PP) at 12 months3
- Secondary endpoints: PP at 6 and 24 months; freedom from Target Lesion Revascularization (FTLR) at 6, 12 and 24 months; technical success; puncture site complication rate; stent fracture rate at 12 and 24 motnhs; clinical success at 6, 12 and 24 months
- Follow-up2: 6, 12 and 24 months
- BIOTRONIK devices: Fortress, Astron Pulsar, Pulsar-18
- Participating centers: Dr. P Peeters, Bonheiden, Belgiumn P.I. Dr. O d‘Archambeau, Antwerp, Belgiumn P.I. Prof. D Scheinert, Leipzig, Germanyn P.I. Prof. G Torsello, Münster, Germany
Results
12-month fracture rates in perspective

Downloads

Vascular Intervention
Self-expanding StentOne-handed stent release for accurate stent deployment
Vascular Intervention
Self-expanding StentTri-axial shaft for a stable delivery system during stent deployment
Sources:
1 Bosiers M, Deloose K, Callaert J, et al. 4-French–compatible endovascular material is safe and effective in the treatment of femoropopliteal occlusive disease: results of the 4-EVER trial. J Endovasc Ther. 2013; 20: 746–756.
2 If patients received both, an Astron Pulsar and Pulsar stent.
3 PSVR < 2.5 by Duplex Ultrasound.
4 Durability II, Supera, Stroll, Zilver PTX.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
