BIOLUX P-II
NCT01867736
First-in-human study to assess the safety and performance of the Passeo-18 Lux drug-coated balloon vs. the uncoated Passeo-18 balloon catheter in patients with stenosis and occlusion of the infrapopliteal arteries
Conclusion
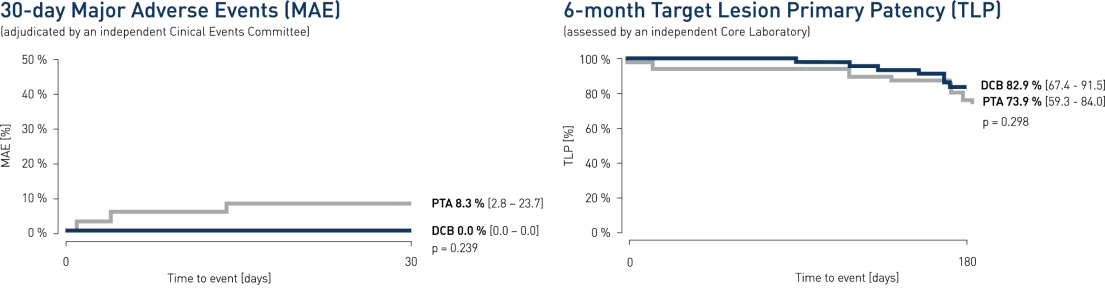
- At 30 days, clinical results show major adverse event (MAE) composite of 0% for the Passeo-18 Lux drug-coated balloon (DCB) vs. 8.3% compared to the control percutaneus transluminal angioplasty (PTA) balloon
- At 6 months angiographic follow-up, Passeo-18 Lux demonstrated a target lesion primary patency (TLP) of 82.9% vs. 73.9% compared to the control PTA balloon
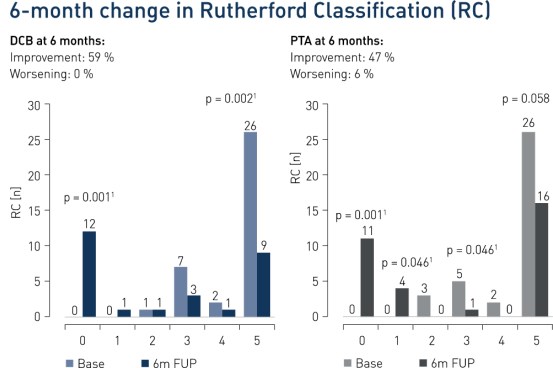
- At 6 months, 59% of patients improved in Rutherford Classification in the DCB group vs. 47% in the control group. Improvement of Rutherford Class 5 patients at 6 months was significant in the DCB group (p = 0.0021)
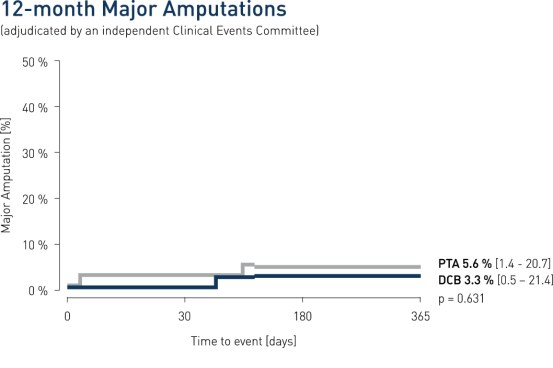
- The Passeo-18 Lux is safe in infrapopliteal lesions, as demonstrated by a low amputation rate and no additional amputations beyond 180 days
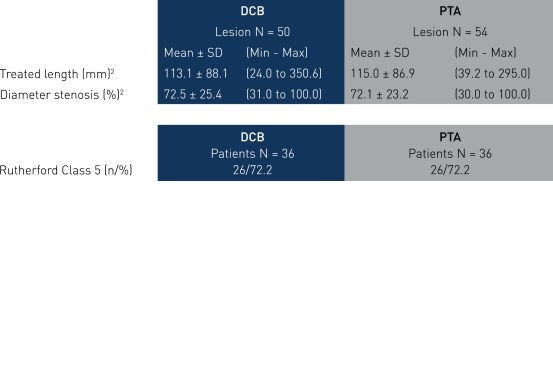
Key Baseline Demographics
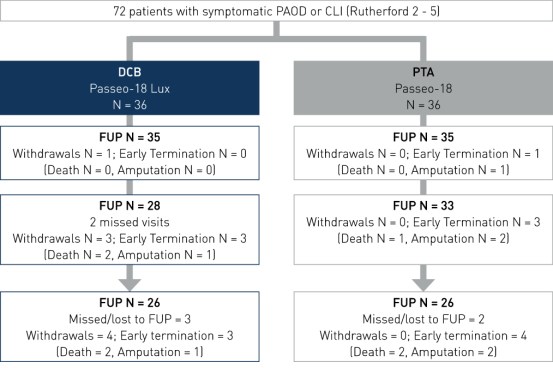
Study Design
- Prospective, multi-center, randomized controlled, first-in-human study
- Number of patients (n): 72
- Principal investigator: Dr. Thomas Zeller, Universitäts-Herzzentrum Freiburg, Bad Krozingen, Germany
- Primary endpoint: 30-day MAE rate2, 6-month TLP measured by quantitative vascular angiography (QVA)3
- Secondary endpoints: 6-month change in Rutherford Class and 12-month major amputation rate
Results
Downloads
Vascular Intervention
Drug-Coated BalloonClinically proven to reduce restenosis and the need for reinterventions1

Vascular Intervention
PTA BalloonHighly pushable coaxial shaft design for most distal lesion access
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
1 p < 0.05 = significant
3 Assessed by an independent core laboratory. PAOD = peripheral arterial occlusive disease; CLI = critical limb ischemia; FUP = follow up; ABI = ankle brachial index; SD = standard deviation
2 MAE = all cause death, major amputation of target extremity, TLR, TVR, target lesion thrombosis, adjudicated by an independent clinical events committee.
