BIOSCIENCE
NCT01443104
Orsiro Sirolimus-eluting stent with biodegradable polymer vs Xience Prima everolimus-eluting stent
Conclusion
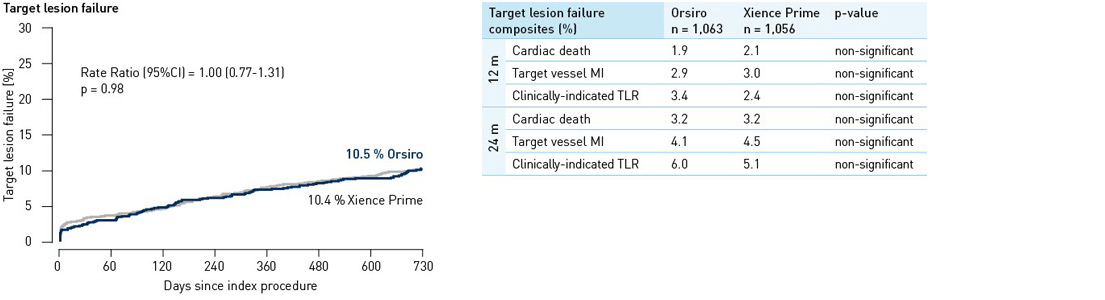
- In this 2,119 patient, randomized, all-comers trial, Orsiro demonstrated non-inferiority to Xience Prime for the primary endpoint at 12 months. A similar trend was shown throughout 24 months.
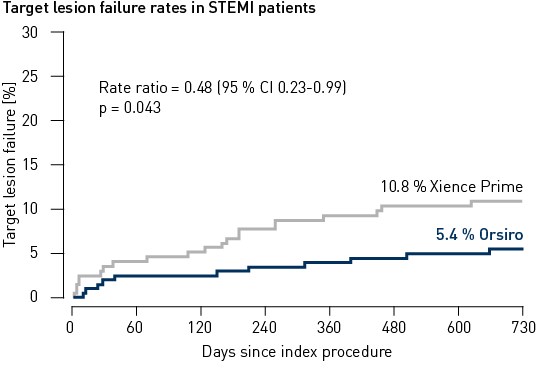
- Orsiro, with its ultrathin struts and bioabsorbable polymer additionally presents superior results in the high-risk subgroup of patients presenting with ST-elevation myocardial infarction (STEMI) out to 24 months.
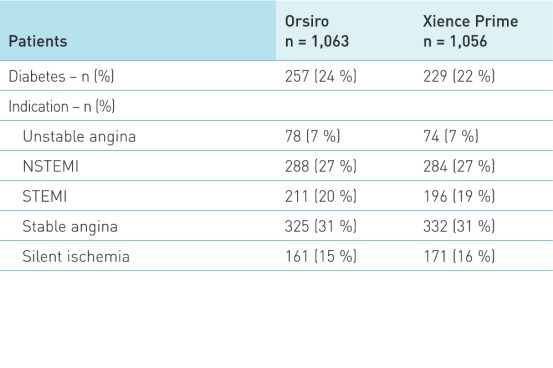
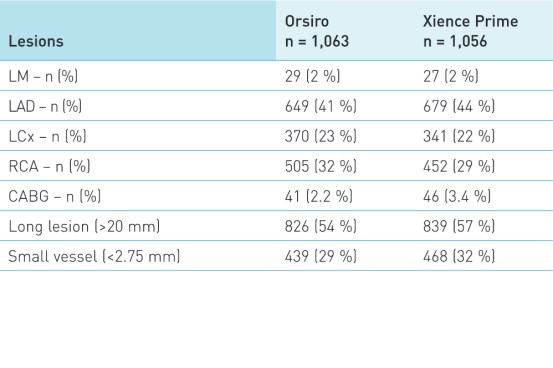
Patient and lesion characteristics1
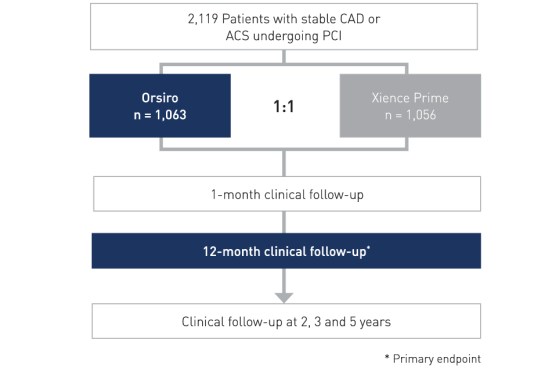
Study Design
- Prospective, all-comers, multicenter, randomized, non-inferiority design
- Principal Investigator: Prof. Stephan Windecker, Bern, Switzerland
Clinical results up to 24 months2
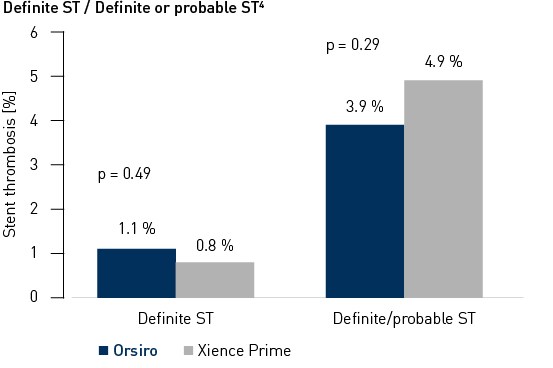
Stent thrombosis up to 24 months2
STEMI subgroup up to 24 months3
Downloads

Disclaimer
© BIOTRONIK AG – All rights reserved. Specifications are subject to modification, revision and improvement. excellence for life
1 Pilgrim T. et al., Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial, Lancet. 2014 Sept 1 (online).
3 Piccolo R. Biodegradable polymer Sirolimus-eluting stents vs. durable polymer Everolimus-eluting stents in patients with STEMI: Two-year follow-up of the BIOSCIENCE. EuroPCR 2016. Oral presentation.
2 Iglesias J., How the Orsiro DES performs in high-risk subgroups, oral presentation at EuroPCR 2015.
4 Definite and probable stent thrombosis according to ARC definition and adjudicated by independent clinical events committee.
