ECOST
Effectiveness and Costs of ICD Follow-up Schedule with Telecardiology
Laurence Guédon-Moreau, European Heart Journal 2013
Study Design
- Randomized, interventional, multicenter study
- Compares the safety of continuous BIOTRONIK Home Monitoring versus conventional ambulatory follow-up of ICDs
- 433 patients at 43 centers in France
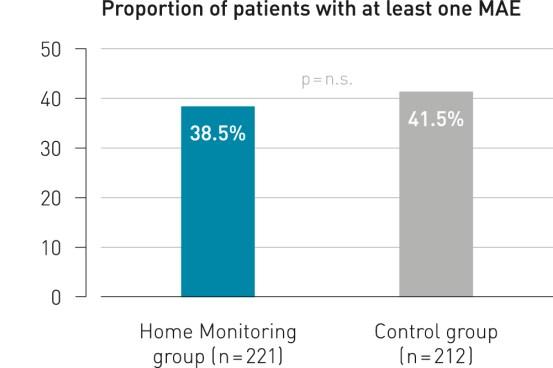
Key Result 1
Remote follow-up with BIOTRONIK Home Monitoring® is as safe as conventional ambulatory follow-up.
Clinical Relevance
- The ECOST study shows that BIOTRONIK Home Monitoring of ICDs is a safe alternative to in-office follow-ups. It positively impacts patient outcome, specially when it comes to reducing the risk of inappropriate shocks – a very unpleasant experience
- The value of BIOTRONIK Home Monitoring for continuous remote monitoring, through its daily transmissions, is essential for clinics to better manage their patients health condition
| Study Objective |
|
|---|---|
| 1° Endpoints |
|
| 2° Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Inclusion Criteria |
|
| Main Exclusion Criteria #1 |
|
| Main Exclusion Criteria #2 |
|
| Devices |
|
| Study Flowchart |
 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
Download Section
Related Products
Tachycardia Therapy
BIOTRONIK offers an extensive product portfolio in the area of tachycardia therapy.


Cardiac Remote Monitoring
BIOTRONIK offers Home Monitoring for its complete product portfolio.
