ENERGY Registry
NCT01056120
Registry to evaluate the clinical performance of the PRO-Kinetic Energy BMS in a large real-world patient population.
Conclusion
- The ENERGY population reflects real-world conditions with bare metal stents (BMS) used mainly in simple lesions.
- In this setting, percutaneous coronary intervention using a cobalt chromium thin-strut BMS with a passive coating showed very good results up to 24 months.
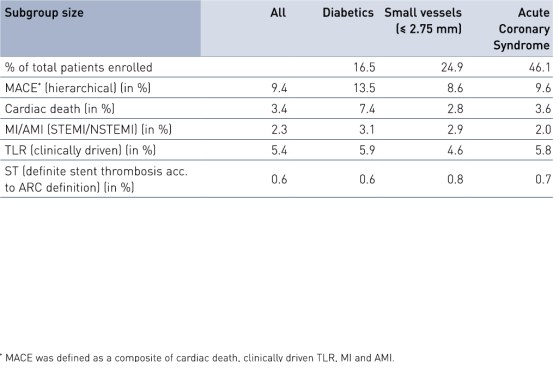
- Results were encouraging with a low composite rate of cardiac death, myocardial infarction (MI) and clinically-driven Target Lesion Revascularization (cd-TLR), even in the pre-defined high-risk groups of diabetes, stents ≤ 2.75 mm and acute coronary syndrome.
Study Design
Prospective, non-randomized, multi-center, observational registry to evaluate the clinical performance of the PRO-Kinetic Energy BMS in a large real-world patient population in standard clinical care.
- Number of patients (n): 1016
- Principal Investigator: Dr. Raimund Erbel, Department of Cardiology, University of Duisburg-Essen, Germany
- Primary endpoint: MACE (Major Adverse Cardiac Events) - composite of cardiac death, cd-TLR, MI and acute myocardial infarction (AMI) at 6 months
- Secondary clinical endpoints (selected): MACE at 12 and 24 months, stent thrombosis (ST) at 6, 12 and 24 months according to ARC definition
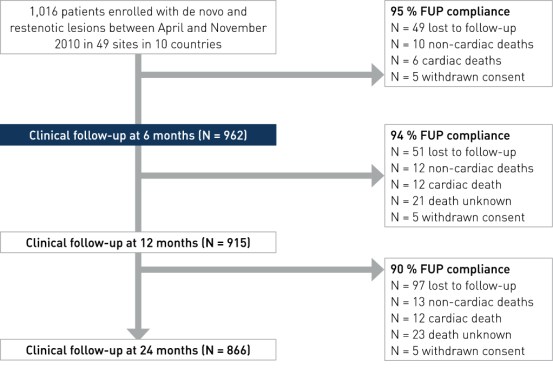
Primary and main secondary endpoint results at 6, 12 and 24 month
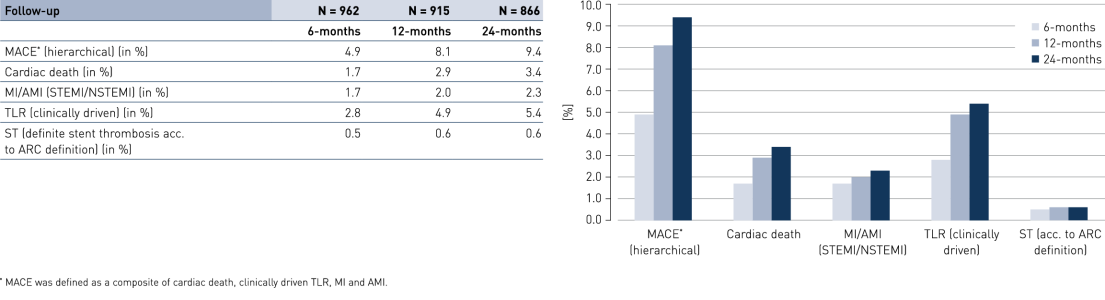
Comparison of subgroups results at 24 months
Downloads

Vascular Intervention
Cobalt Chromium Coronary Stent System60 μm thin struts for better clinically proven results
Vascular Intervention
Clinical StudyA randomized trial of paclitaxel-eluting balloon after bare metal stent implantation vs. bare metal stent in STEMI
Source:
Erbel R, Eggebrecht H, Roguin A, et al. Prospective, multi-center evaluation of a silicon carbide coated cobalt chromium bare metal stent for percutaneous coronary interventions: Two-year results of the ENERGY Registry. Cardiovasc Revasc Med. 2014; 15 (11): 381–387.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
