IN-TIME
Influence of Home Monitoring on the Clinical Status of Heart Failure Patients
Hindricks et al., The Lancet 2014
Study Design
- Randomized, controlled, international, multicenter study
- Assesses the impact of BIOTRONIK Home Monitoring® on the clinical status of heart failure patients
- 664 patients at 36 centers
Key Result 1
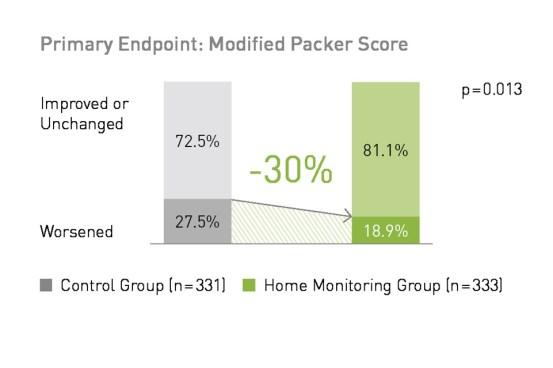
At 12 months follow-up, significantly fewer patients in the BIOTRONIK Home Monitoring® group worsened according to the modified Packer score compared to the control group (18.9% vs27.5%; p<0.05).
Key Result 2
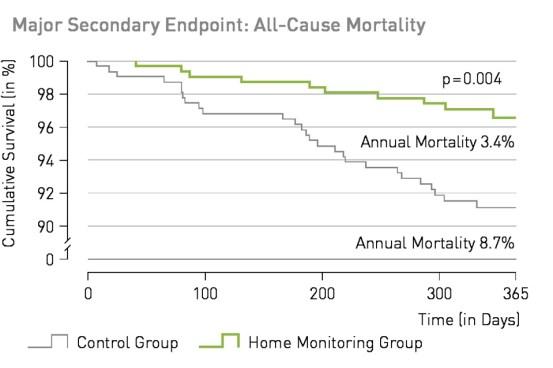
Significant reduction of all-cause mortality in heart failure patients of more than 60% and beneficial effect on the composite clinical score with implant-based (ICD or CRT-D) BIOTRONIK Home Monitoring® compared to standard care.
Clinical Relevance
- The IN-TIME study demonstrated improved clinical status for heart failure patients by implementing a remote monitoring system based on:
- A reliable transmission rate of 85%
- A clinically relevant set of rhythmological and technical parameters
- Clinical workflow that enables fast patient contact and follow-up within one to two working days
- The European Society of Cardiology added a clear recommendation for the use of implant-based remote follow-up to its guidelines1
| Study Objective |
|
|---|---|
| 1° Endpoints |
|
| 2° Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Devices |
|
| Flowchart |
 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
Download Section
Related Products
Cardiac Resynchronization Therapy
BIOTRONIK offers an extensive product portfolio in the area of Cardiac Resynchronization.

Cardiac Remote Monitoring
BIOTRONIK offers Home Monitoring for its complete product portfolio.

1 Brignole M et al. 2013 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. European Heart Journal. 2013, 34(29).
