BIOFLOW-III
Clinical Study
Investigating Orsiro drug-eluting coronary stent
Conclusion
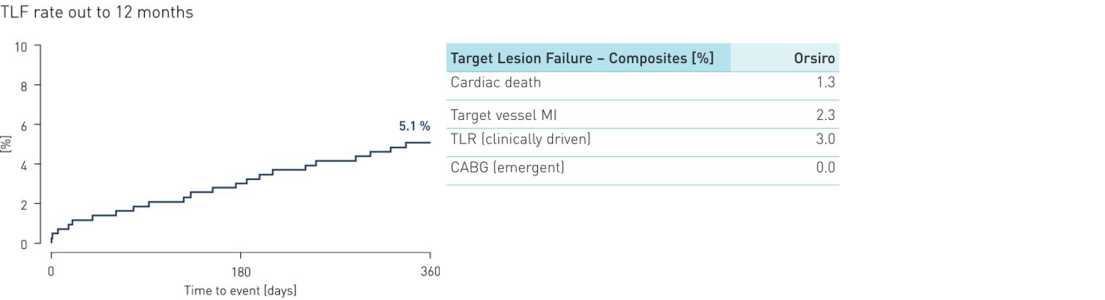
- In this all-comers setting a low TLF rate of 5.1 % was observed within the first 12 months, which demonstrates safety and effectiveness of the Orsiro Hybrid Stent.
- The results observed in this “real world” population demonstrate a low TLF rate comparable to other state of the art DES at 12 months.
- In comparison with other published trials in patients with STEMI, Orsiro shows a low TLF rate.
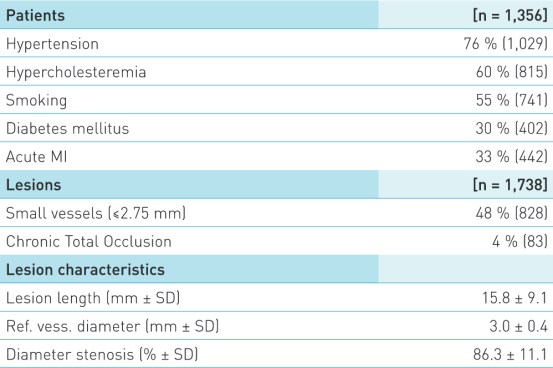
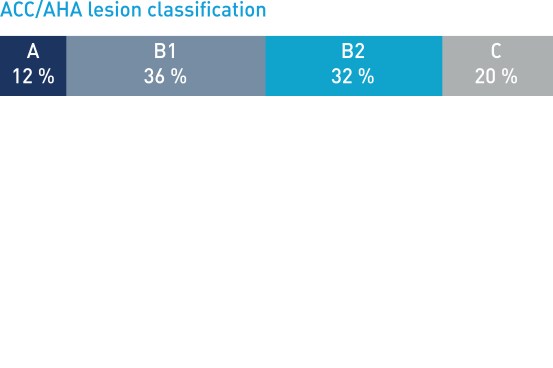
Patient and lesion characteristics
圖片
圖片
Study Design
International, prospective, multi-center open-label, registry of the Orsiro drug-eluting stent in daily clinical practice.
- Coordinating clinical investigator: Prof. Dr. Johannes Waltenberger, Universitätsklinikum Münster, Germany
- Pre-defined sugroups: Diabetic patients, Small vessels (≤2.75 mm), Chronic Total Occlusion, Acute MI (STEMI and NSTEMI)
- Post-hoc subgroups: B2/C lesions, Multivessel disease (MVD), STEMI vs NSTEMI
圖片
Primary endpoint results
圖片
Downloads

Disclaimer
© BIOTRONIK AGAll rights reserved. Specifications are subject to modification, revision and improvement.
