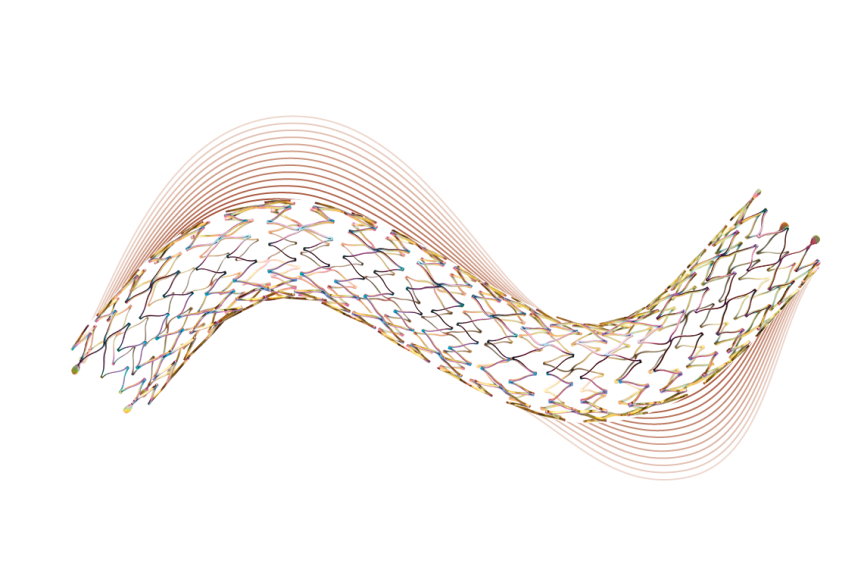
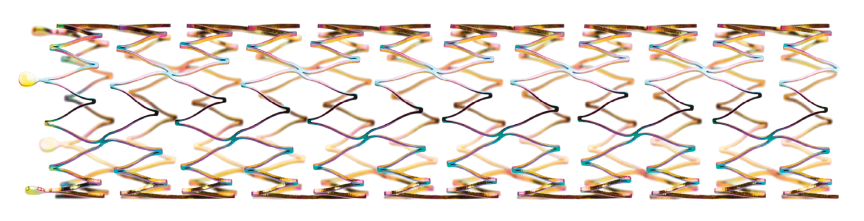
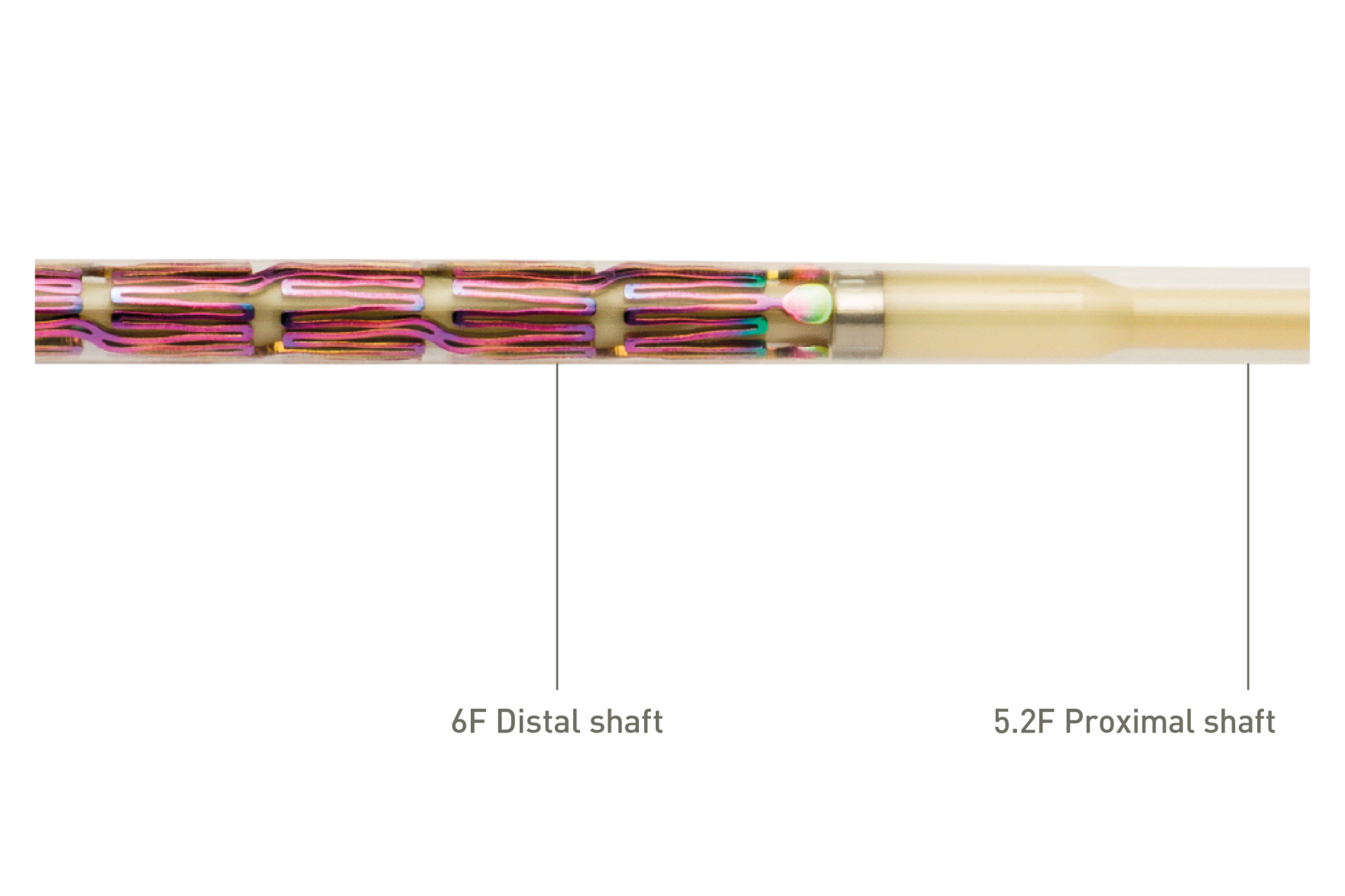
Gesegmenteerd stentontwerp en stutdikte om voldoende chronische buitenwaartse kracht in het iliacale gebied te bieden, terwijl het piek-tot-dal-ontwerp en de S-scharnierende verbindingsstaven directionele flexibiliteit bieden en visschubben in kronkelige slagaders vermijden
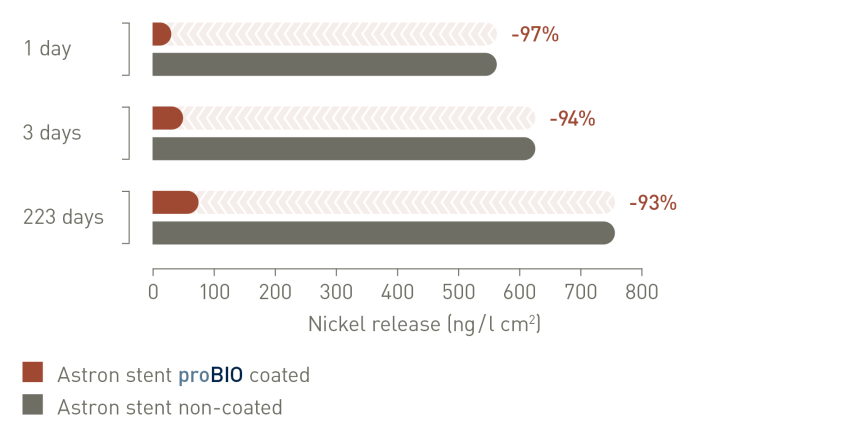
proBIO® siliciumcarbidecoating vermindert de ionenafgifte door te fungeren als een effectieve en betrouwbare barrière tegen diffusie van nikkel en andere zware metalen2