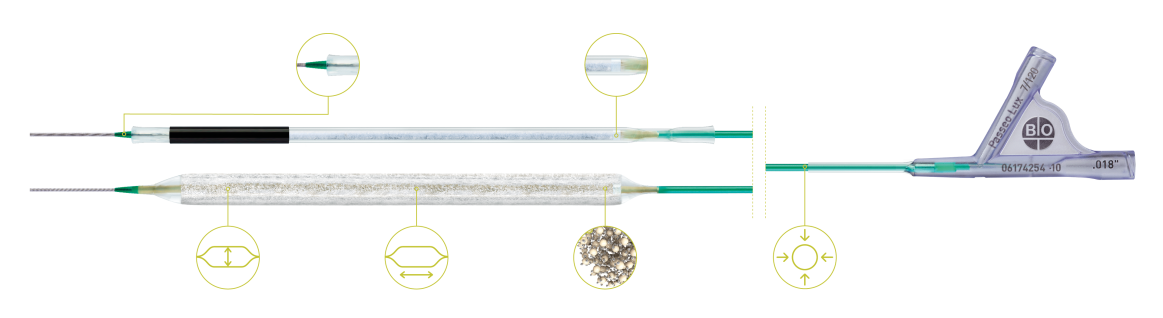
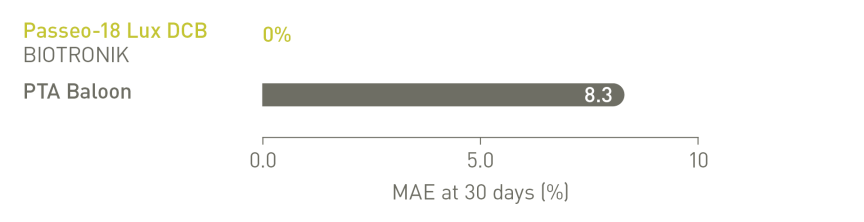BIOLUX P-I RCT² femoropopliteale indicatie¹⁰
Aanzienlijk verminderde revascularisatie van doellaesies (TLR) na 12 maanden vergeleken
met de controle-PTA*-ballon in de as-treated populatie²
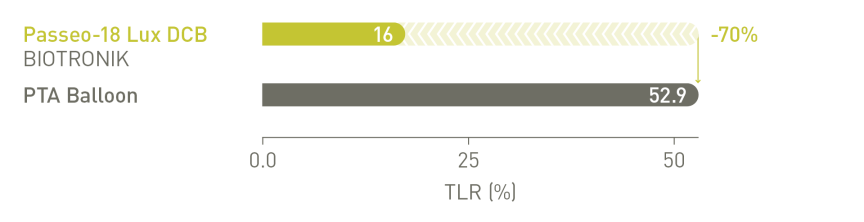
BIOLUX P-II RCT³ infrapopliteale indicatie
De frequentie ernstige bijwerkingen (MAE) van de Passeo-18 Lux DCB was lager vergeleken met
de controle-PTA-ballon.
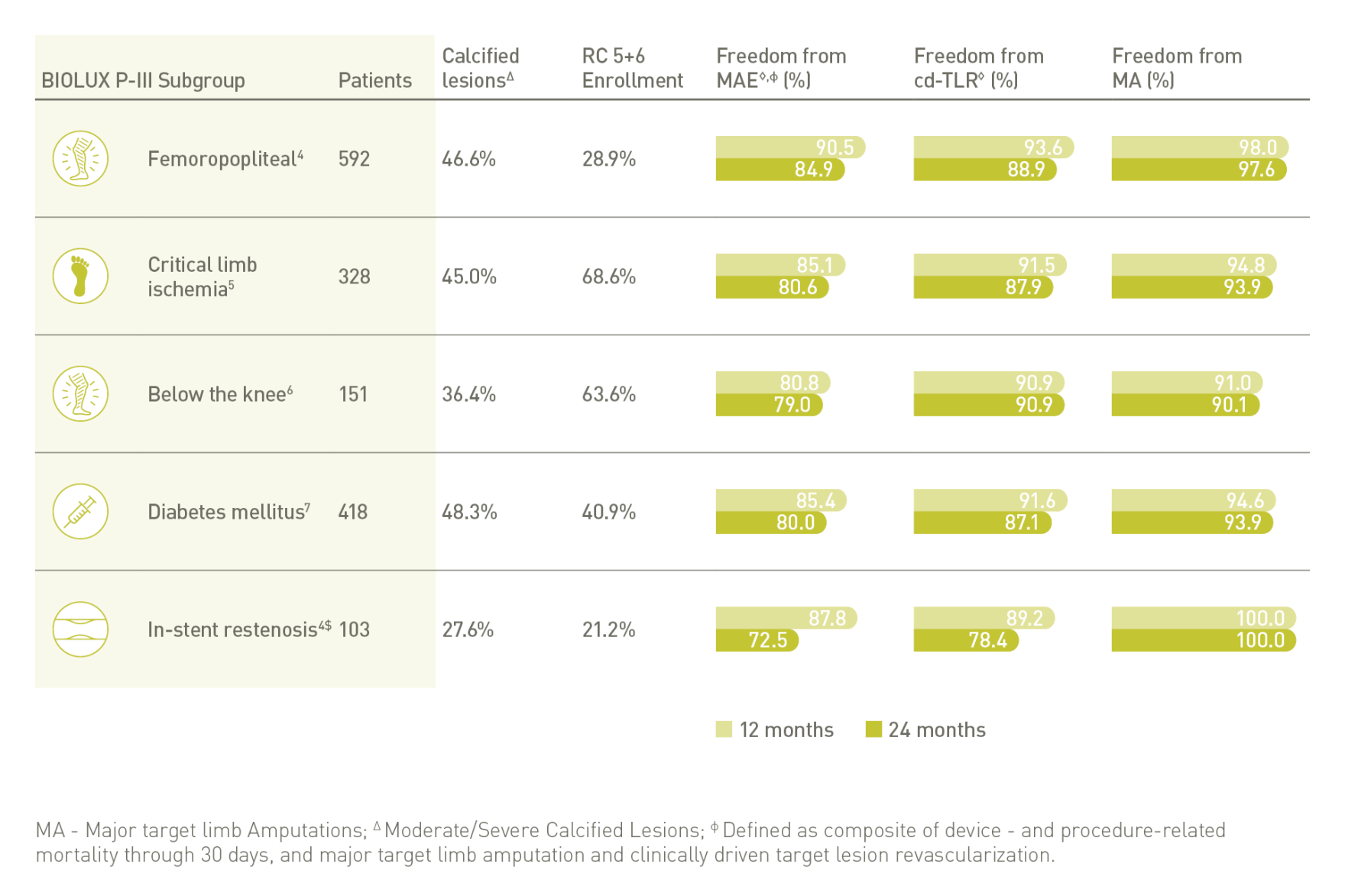
BIOLUX P-III⁴ all-comers-register
Passeo-18 Lux DCB laat uitstekende resultaten zien in één van de grootste DCB-registers ter wereld met weinig uitsluitingscriteria.