EchoCRT
Cardiac Resynchronization Therapy in Heart Failure with a Narrow QRS Complex
Ruschitzka et al., The New England Journal of Medicine 2013
Study Design
- Randomized, prospective, parallel, double-blinded, multi-center, international trial
- Evaluates the effects of CRT on mortality and morbidity of subjects with heart failure due to left ventricular systolic dysfunction with a narrow QRS width (
- 1,680 enrolled, 809 randomized (404 patients to CRT and 405 to control) at 115 sites in 17 countries
Key Result 1
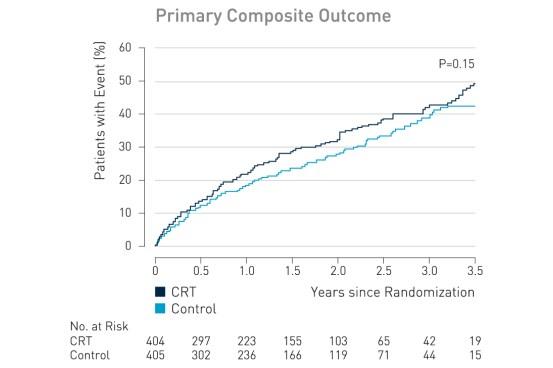
The primary outcome, death from any cause or hospitalization for worsening heart failure, occurred in 116 of 404 patients (28.7%) in the CRT group, as compared with 102 of 405 (25.2%) in the control group (hazard ratio with CRT, 1.20; 95% confidence interval [CI], 0.92 to 1.57; P = 0.15).
Key Result 2
The rate of freedom from complications related to the CRT-D system at 6 months was 89.6% for the population that included all patients who underwent an attempted implantation.1
Clinical Relevance
- As compared with an ICD with inactivated CRT, a CRT-D did not reduce the rate of death from any cause or hospitalization for heart failure and may increase mortality among patients with systolic heart failure and a QRS duration of less than 130 msec¹
- Our results reinforce the notion that, at least until new methods of assessment are developed, QRS width (with or without mechanical dyssynchrony) remains the primary determinant of response to CRT¹
| Study Objective |
|
|---|---|
| 1° Endpoints |
|
| 2° Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Key Inclusion Criteria |
|
| Key Exclusion Criteria |
|
| Devices |
|
| Follow-Up |
|
| Study Duration |
|
| Reference No. |
|
| Principal Investigators |
|
Download Section
Disclaimer
On March 13, 2013, the study was stopped on the recommendation of the data and safety monitoring board, on the basis of futility with a potential for harm.
1 Ruschitzka F, Abraham W, Singh J, Bax J, Borer J, Brugada J, Dickstein K, Ford I, Gorcsan J III, Gras D, Krum H, Sogaard P, Holzmeister J; Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex, New England Journal of Medicine, 2013; 369:1395-1405.
