Pantera®LEO
Non-Compliant High Pressure Balloon Catheter
Indicated for stent post-dilatation and dilatation of a coronary artery or bypass graft stenosis.a
Product Highlights
Image
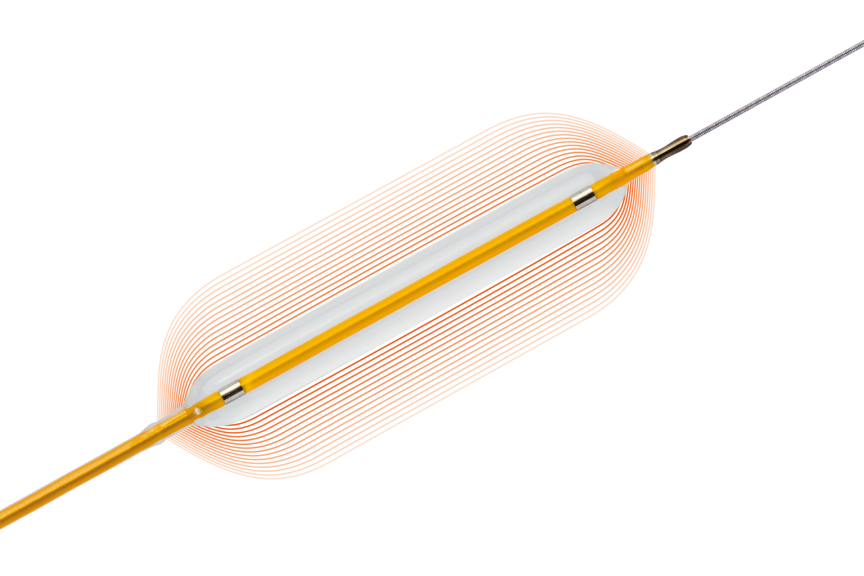
Lowest compliance in classb avoiding dog-bone effect1
More controlled growth from NP to RBP than leading competitorsc1
Image
Precise dilatation1
Extra short balloon shoulders reduce longitudinal balloon growth1
Image
Enhanced crossability1
Patchwork coating for enhanced crossability1
Technical Data
| Proximal shaft | |
|---|---|
| Design | Hypotube design |
| Diameter | 2.0F |
| Shaft markers | 92 cm and 102 cm from tip |
| Coating | Hydrophobic |
| Distal shaft | |
| Guiding catheter | 5F (min. I.D. 0.056”) |
| Guide wire diameter | 0.014” |
| Lesion entry profile | 0.018” |
| Usable length | 145 cm |
| Distal shaft length | 34 cm |
| Balloon material | SCP (Semi Crystalline Polymer) |
| Balloon folding | 3-fold |
| Balloon markers | Platinum-Iridium |
| Coating | Hydrophilic (end of balloon to guide wire exit port); hydrophobic (balloon and tip) |
| Diameter | 2.6F (ø 2.0 - 3.75 mm); 2.7F (ø 4.0 - 5.0 mm) |
Technical Data
| Proximal shaft | |
|---|---|
| Design | Hypotube design |
| Diameter | 2.0F |
| Shaft markers | 92 cm and 102 cm from tip |
| Coating | Hydrophobic |
| Distal shaft | |
|---|---|
| Guiding catheter | 5F (min. I.D. 0.056”) |
| Guide wire diameter | 0.014” |
| Lesion entry profile | 0.018” |
| Usable length | 145 cm |
| Distal shaft length | 34 cm |
| Balloon material | SCP (Semi Crystalline Polymer) |
| Balloon folding | 3-fold |
| Balloon markers | Platinum-Iridium |
| Coating | Hydrophilic (end of balloon to guide wire exit port); hydrophobic (balloon and tip) |
| Diameter | 2.6F (ø 2.0 - 3.75 mm); 2.7F (ø 4.0 - 5.0 mm) |
Compliance Chart
| Balloon diameter x length (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ø 2.00 x 8-30 |
ø 2.25 x 8-30 |
ø 2.50 x 8-30 |
ø 2.75 x 8-30 |
ø 3.00 x 8-30 |
ø 3.25 x 8-30 |
ø 3.50 x 8-30 |
ø 3.75 x 8-30 |
ø 4.00 x 8-30 |
ø 4.50 x 8-30 |
ø 5.00 x 8-30 |
||
| Nominal Pressure (NP) |
atm* | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
ø (mm) |
2.00 |
2.25 |
2.50 |
2.75 |
3.00 |
3.25 |
3.50 |
3.75 |
4.00 |
4.50 |
5.00 |
|
| Rated Burst Pressure (RBP) |
atm* | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 18 | 18 |
ø (mm) |
2.05 |
2.32 |
2.57 |
2.83 |
3.09 |
3.35 |
3.61 |
3.89 |
4.12 |
4.56 |
5.07 |
|
| *1 atm = 1.013 bar | ||||||||||||
Ordering Information
| Balloon ø (mm) |
Catheter length 145 cm Balloon length (mm) |
|||||
|---|---|---|---|---|---|---|
| 5F | 8 | 12 | 15 | 20 | 30 | |
| 2.00 | 366991 | 367002 | 367013 | 367024 | 367035 | |
| 2.25 | 366992 | 367003 | 367014 | 367025 | 367036 | |
| 2.50 | 366993 | 367004 | 367015 | 367026 | 367037 | |
| 2.75 | 366994 | 367005 | 367016 | 367027 | 367038 | |
| 3.00 | 366995 | 367006 | 367017 | 367028 | 367039 | |
| 3.25 | 366996 | 367007 | 367018 | 367029 | 367040 | |
| 3.50 | 366997 | 367008 | 367019 | 367030 | 367041 | |
| 3.75 | 366998 | 367009 | 367020 | 367031 | 367042 | |
| 4.00 | 366999 | 367010 | 367021 | 367032 | 367043 | |
| 4.50 | 367000 | 367011 | 367022 | 367033 | 367044 | |
| 5.00 | 367001 | 367012 | 367023 | 367034 | 367045 | |
Downloads and Related Links
Downloads
Related Links
How can we help you?
References
a. Indication as per IFU; b. When compared to main competitors, compliance curves 3.0 mm balloons; c. NP-RBP growth for 3.0 diameters. Leading competitors have been selected based on the PTCA Balloon Catheter Revenue Market Shares US, 2016; (Source: Milennium Research Group Inc.). Latest NC balloons for each competitor.
1. BIOTRONIK Data on file.
Pantera is a trademark or registered trademark of the BIOTRONIK Group of Companies.