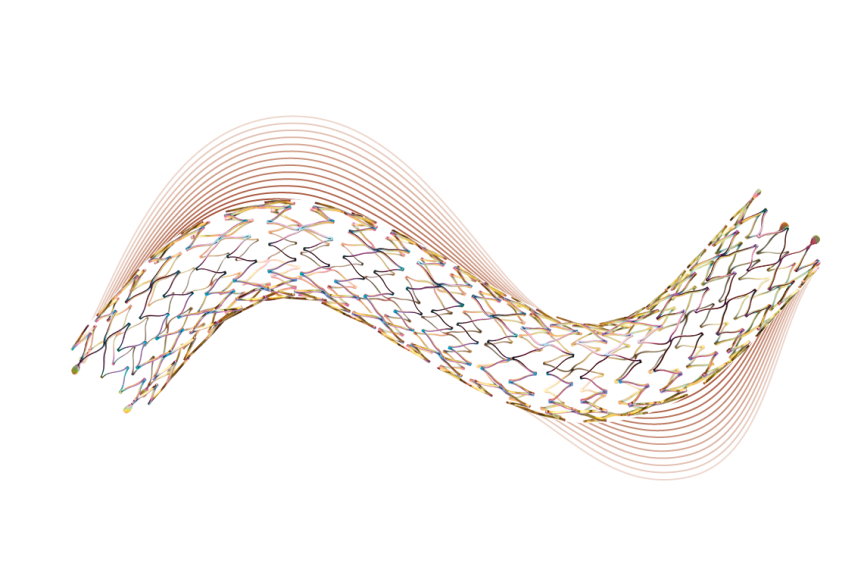
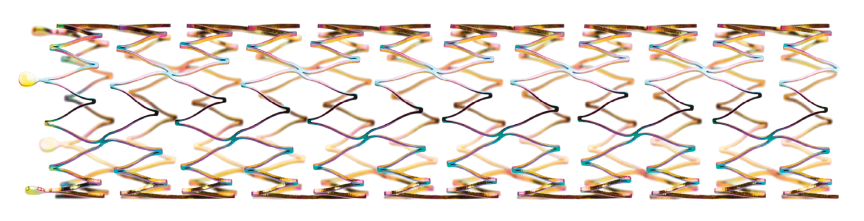
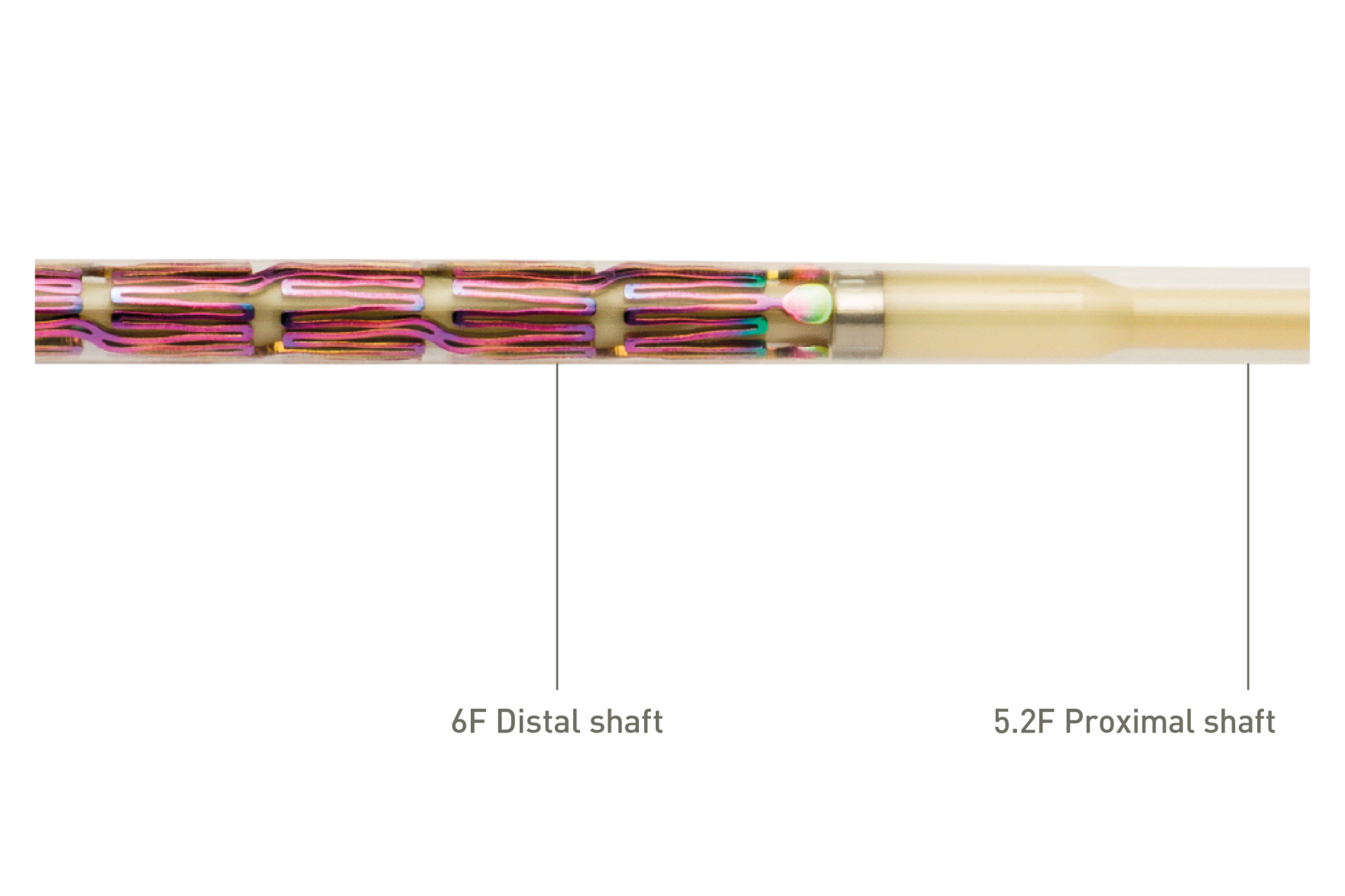
Segmented stent design and strut thickness to provide sufficient chronic outward force in the iliac territory, while the peak-to-valley design and S-articulating connecting bars provide multi-directional flexibility and avoid fish-scaling in tortuous arteries
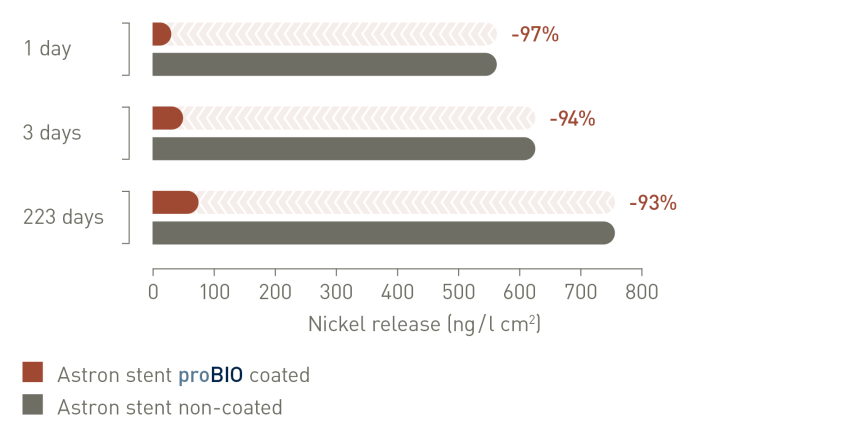
proBIO® silicon carbide coating reduces ion release by acting as an effective and reliable barrier to nickel and other heavy metal ion diffusion2