Dynamic
Stent designed for flexibility and improved stent surface biocompatibility
Indicated for the treatment of de novo or restenotic atherosclerotic lesions in iliac arteries.*
Product Highlights
Image
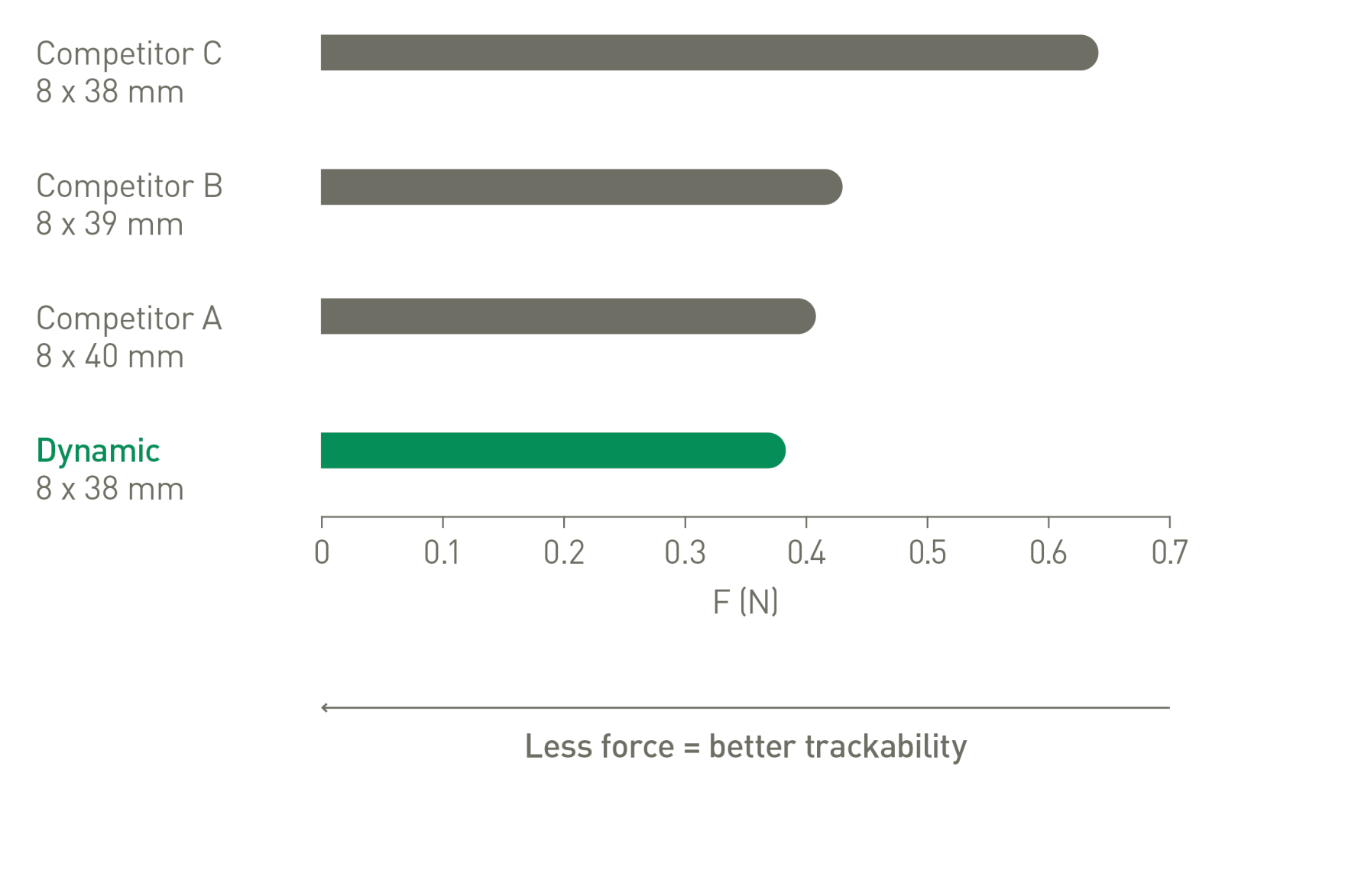
Excellent trackability
Image
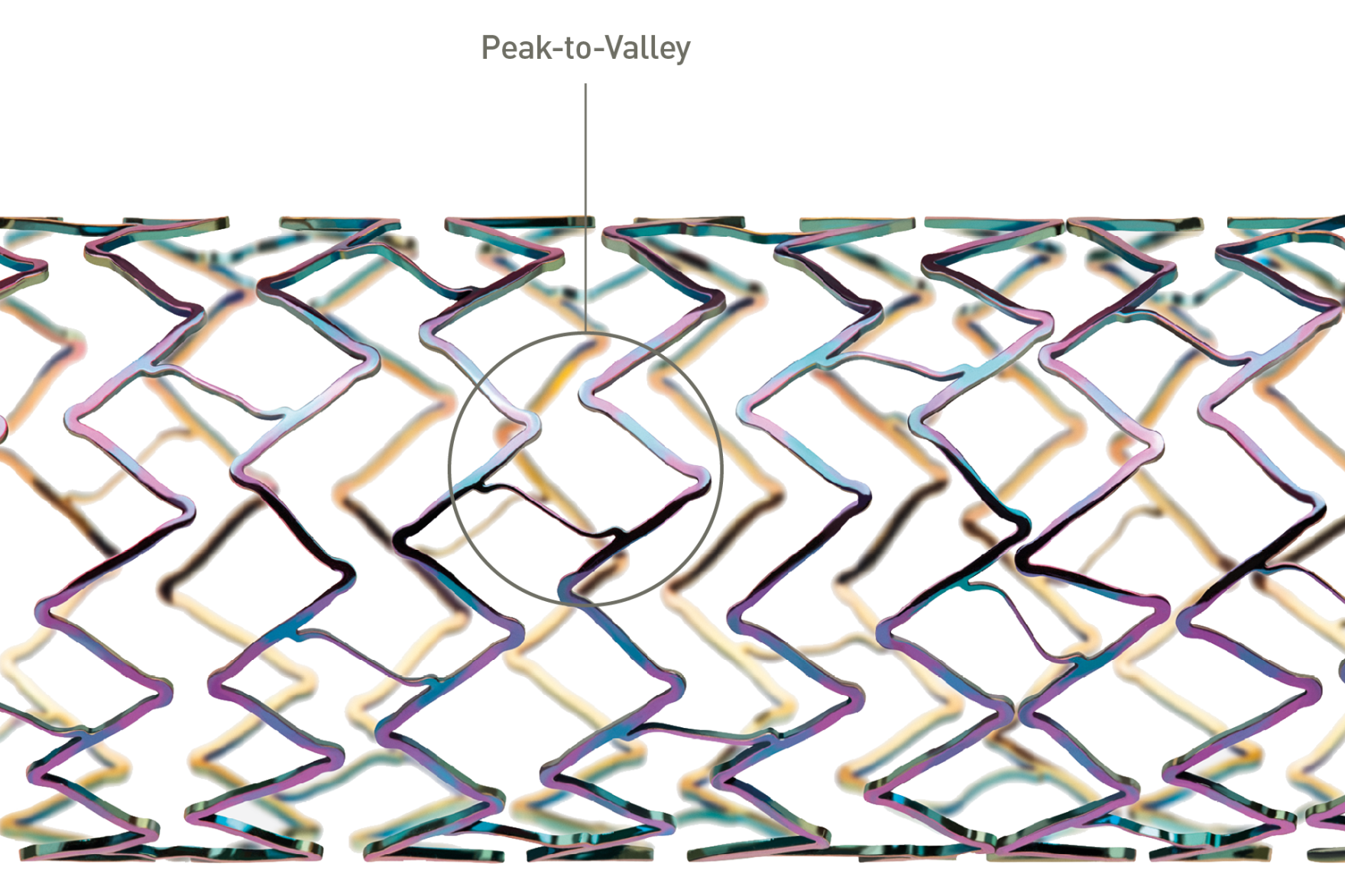
Stent designed for flexibility in iliac arteries
Image
Improved stent surface biocompatibility
Technical Data
| Stent | |
|---|---|
| Stent | Balloon-expandable |
| Stent material | Stainless Steel |
| Strut thickness | 160 µm (ø 5.0 - 8.0 mm) 180 µm (ø 9.0 - 10.0 mm) |
| Shortening | Negligible |
| Stent coating | proBIO® (Amorphous Silicon Carbide) |
| Sizes | ø 5.0 - 10.0 mm; L: 15 - 25 - 38 - 56 mm |
| Delivery system | |
| Catheter type | OTW |
| Recommended guide wire | 0.035” |
| Tip | Soft, short, tapered, colored |
| Balloon markers | 2 swaged markers |
| Shaft | 5F, hydrophobic coating, dual-lumen |
| Usable length | 80 cm and 130 cm (ø 5.0 - 8.0 mm) |
| Markers | 2 swaged markers |
| Guide wire lumen | Hydrophobic coating |
| Nominal Pressure (NP) | 9 atm |
| Rated Burst Pressure (RBP) | 15 atm (ø 5.0 - 8.0 mm) 13 atm (ø 9.0 - 10.0 mm) |
Ordering Information
| Stent ø (mm) |
Catheter length 80 cm Stent length (mm) |
||||
|---|---|---|---|---|---|
| 15 | 25 | 38 | 56 | ||
| 5.0 | 350110(5F) | 350114(5F) | 350120(6F) | 350126(6F) | |
| 6F | 6.0 | 350111 | 350115 | 350121 | 350127 |
| 7.0 | 350112 | 350116 | 350122 | 350128 | |
| 8.0 | 350113 | 350117 | 350123 | 350129 | |
| 9.0 | - | 350118 | 350124 | 350130 | |
| 7F | 10.0 | - | 350119 | 350125 | 350131 |
| Stent ø (mm) |
Catheter length 130 cm Stent length (mm) |
||||
|---|---|---|---|---|---|
| 15 | 25 | 38 | 56 | ||
| 5.0 | 350132(5F) | 350136(5F) | 350140(6F) | 350144(6F) | |
| 6F | 6.0 | 350133 | 350137 | 350141 | 350145 |
| 7.0 | 350134 | 350138 | 350142 | 350146 | |
| 8.0 | 350135 | 350139 | 350143 | 350147 | |
Downloads and Related Links
How can we help you?
References
*Indication as per IFU. Australia: not TGA approved for use within common iliac arteries.
1. BIOTRONIK data on file; 2. Rzany A, Schaldach M. Smart Material Silicon Carbide: Reduced Activation of Cells and Proteins on a-SiC:H-coated Stainless Steel. Progress in Biomedical Research 2001; May: 182- 194.
Dynamic and proBIO are trademarks or registered trademarks of the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owners.