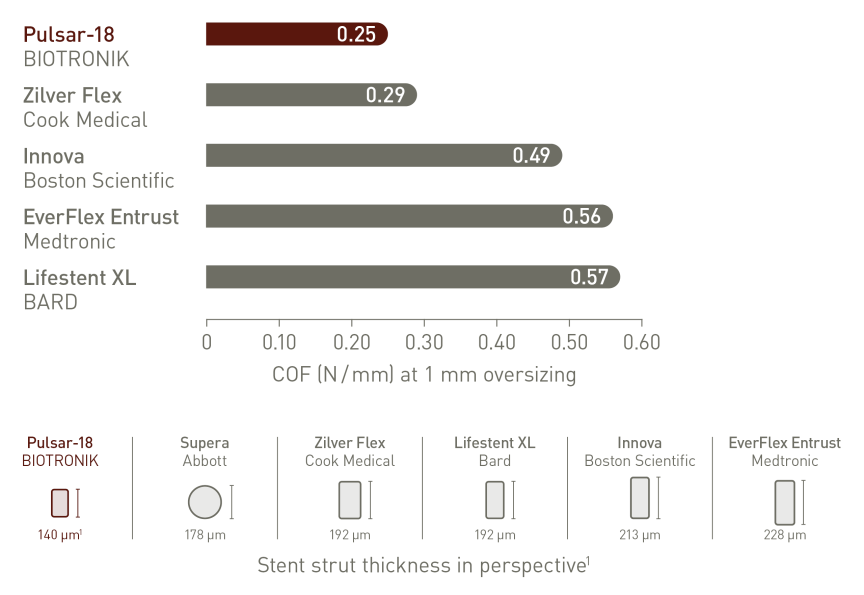
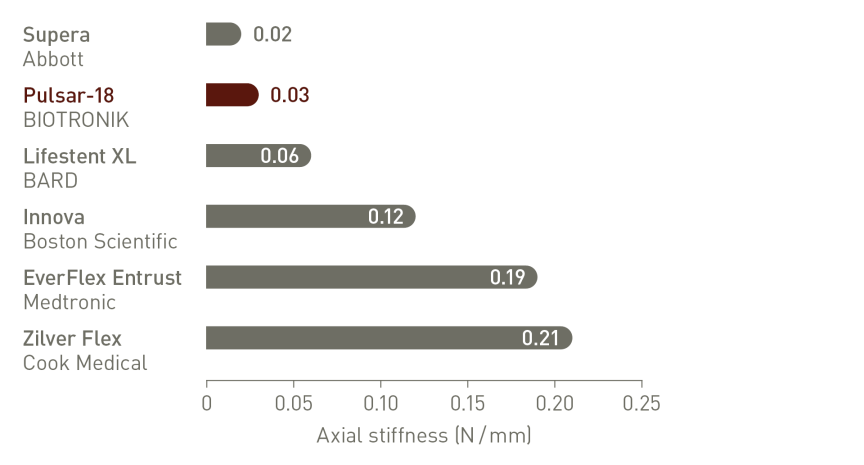
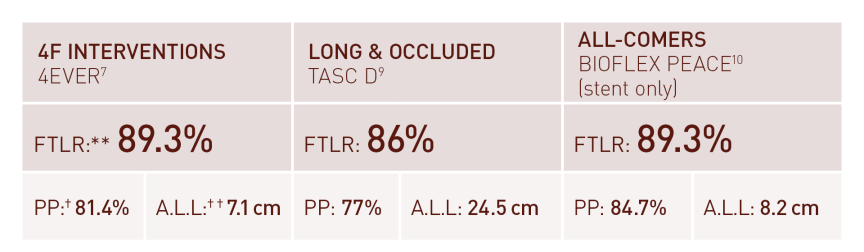
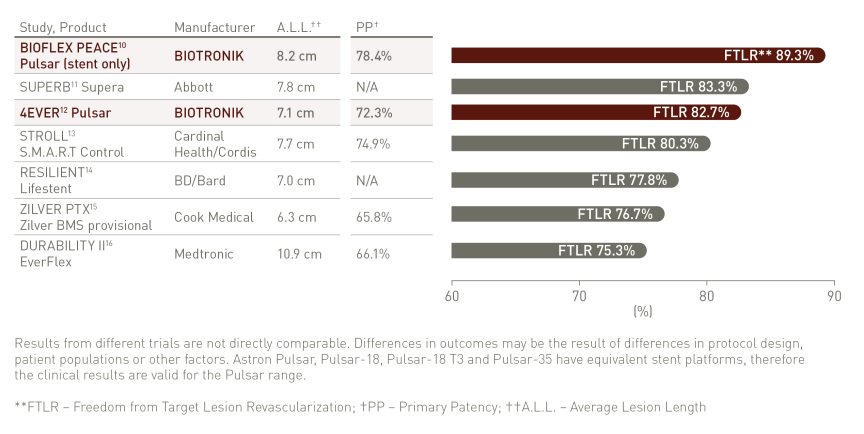
Thinner struts for low Chronic Outward Force (COF)²
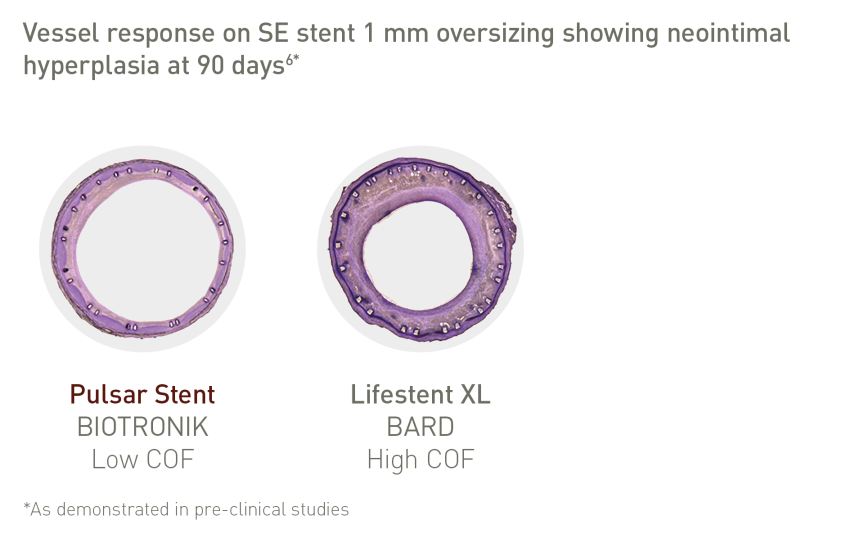
Thinner struts and lower COF make a difference:*
• Lower risk of restenosis3
• Reduced vessel injury and inflammation3
• Faster endothelialization4, 5
Stent designed for SFA*: multi-directional flexibility to conform to the natural vessel movement
*Superficial Femoral Artery