Key Results
Key Result 1
COMPAS demonstrated a 66% reduction in hospitalizations for atrial arrhythmia and related stroke in the Home Monitoring group.
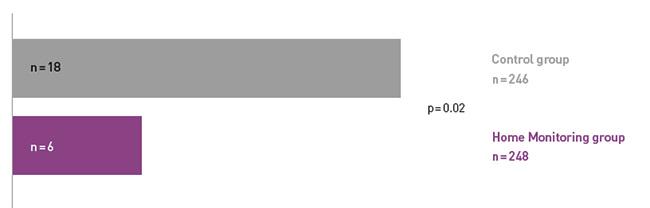
A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial)
MABO P ET AL., EUROPEAN HEART JOURNAL, 2012
COMPAS demonstrated a 66% reduction in hospitalizations for atrial arrhythmia and related stroke in the Home Monitoring group.
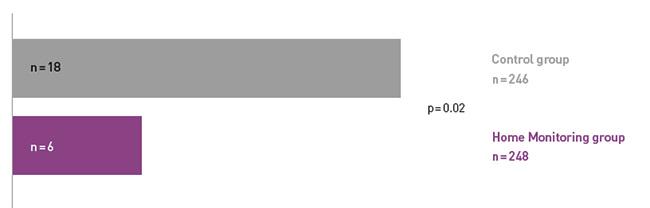
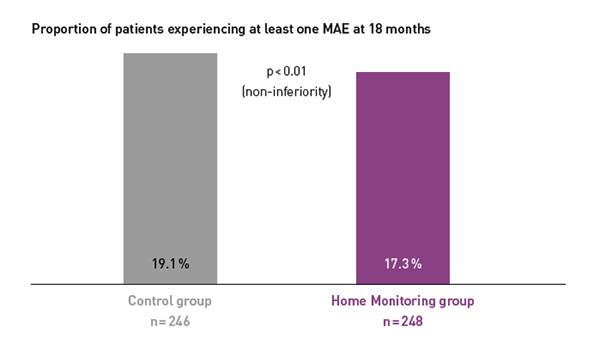
COMPAS showed comparable safety event rates in both groups.
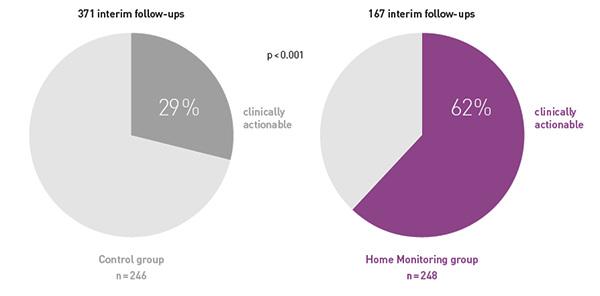
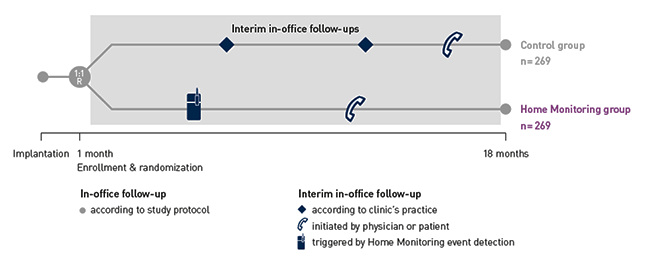
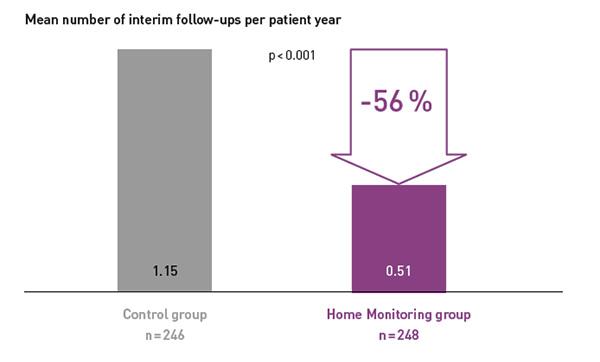
COMPAS demonstrated that BIOTRONIK Home Monitoring® reduced the number of interim in-office follow-ups by 56 %
At the same time, follow-ups were more often clinically actionable, resulting in pacemaker reprogramming or medication changes.