First Outcome Trial on the Benefits of ICMs in Post-MI Patients
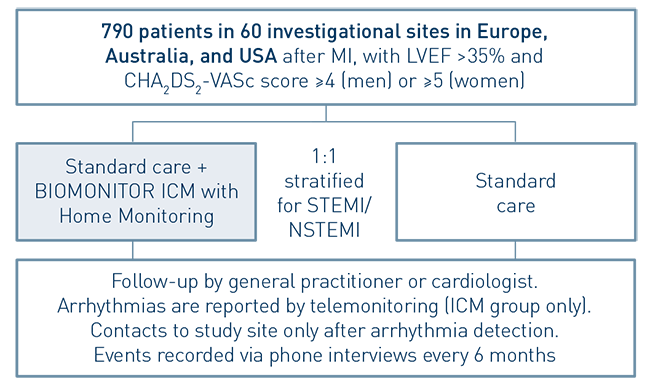
The BIO|GUARD-MI study is the first trial to investigate the impact of continuous arrhythmia monitoring with an implantable cardiac monitor (ICM) on clinical outcomes in post-myocardial infarction (MI) patients. Implantable cardiac monitors are also referred to as implantable loop recorders.
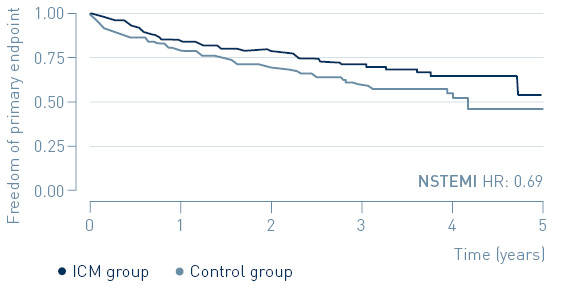
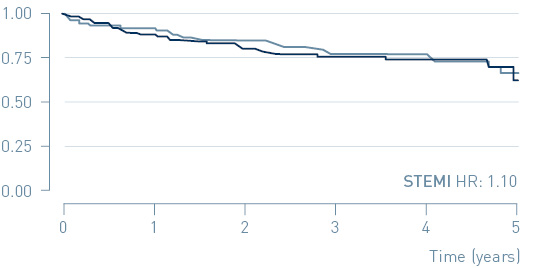
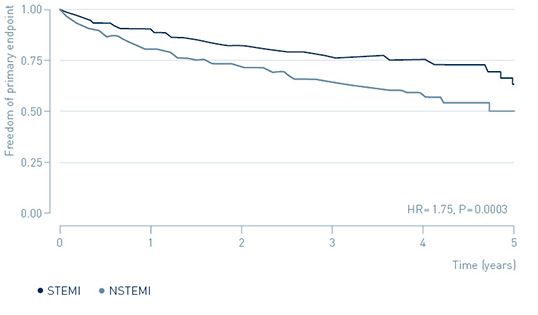
The study demonstrated that early treatment of high-risk, non-ST-segment elevation myocardial infarction (NSTEMI) patients guided by continuous arrhythmia monitoring with implantable loop recorders reduces major adverse cardiac events (MACE) by 31%.
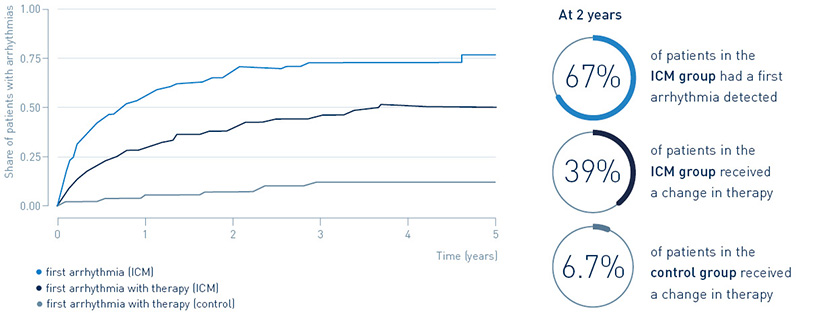
67% of patients with an implantable loop recorder had a first arrhythmia detected after two years, leading to guideline-recommended change in treatment in 39.4%. Only 6.7% of post-MI patients without an implantable loop recorder received a change in treatment.
Read more about the study design, key results, and clinical relevance below.
As presented at a Late-Breaking Clinical Trial session at the American College of Cardiology’s 71st Annual Scientific Session in Washington D.C. on April 4, 2022