Key Results
Key Result 1
Significantly Lower Rate of Major Complications with CRT-DX
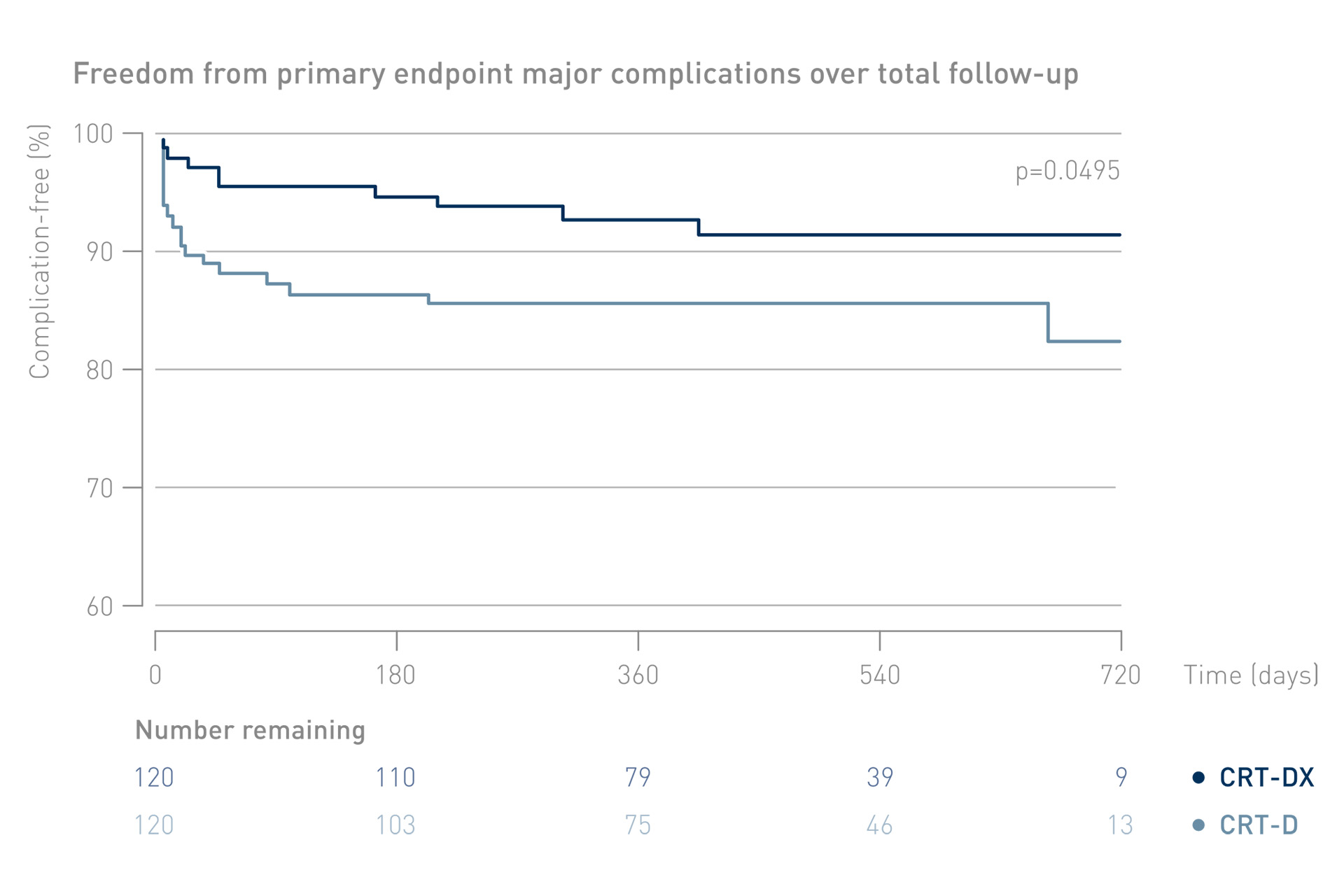
With CRT-DX, significantly fewer patients experience major complications.
Novel Two-Lead Cardiac Resynchronization Therapy System Provides Equivalent CRT Responses with Less Complications than a Conventional Three-Lead System: Results from the QP ExCELs Lead Registry
Shaik N et al., Journal of Cardiovascular Electrophysiology, May 2020.
doi: 10.1111/jce.14552

With CRT-DX, significantly fewer patients experience major complications.
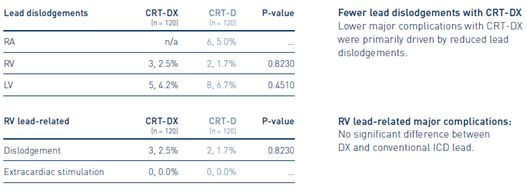
2 Author’s conclusion extracted from publication.
Freedom from major complications, defined as events related or possibly related to the implanted system or the implant procedure and requiring invasive intervention to resolve.
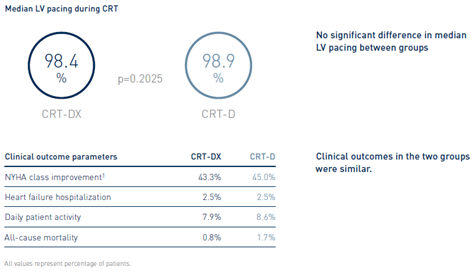
CRT device performance and response:
Mean follow-up
NCT02290028 (QP ExCELs)
1 Brignole M et al. 2013 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. European Heart Journal. 2013, 34(29).