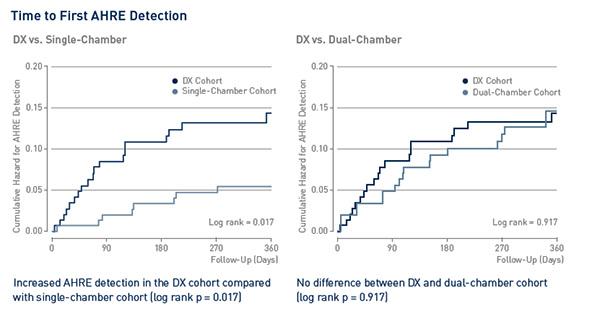
Key Results
Key Result 1

Subclinical atrial fibrillation detection with a floating atrial sensing dipole in single lead implantable cardioverter defibrillator systems: Results of the SENSE trial
THOMAS G ET AL., JOURNAL OF CARDIOVASCULAR ELECTROPHYSIOLOGY 2019
doi: 10.1111/JCE.14081