TASC D II
Evaluation of the 4 French Pulsar-18 self-expanding nitinol stent in long femoropopliteal lesions (TASC D II) – 12-month results
Conclusion
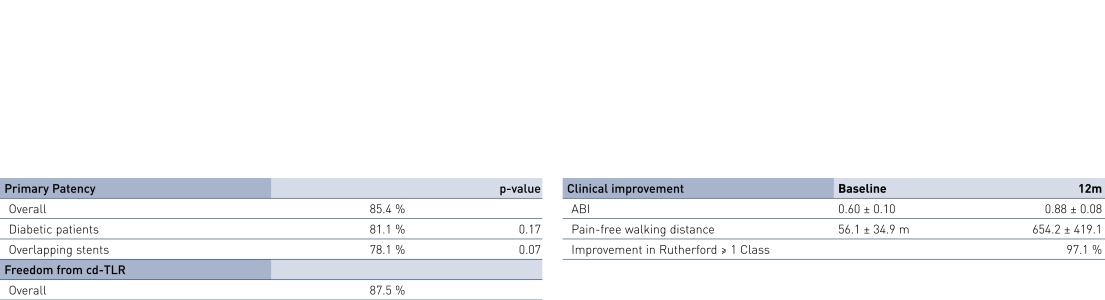
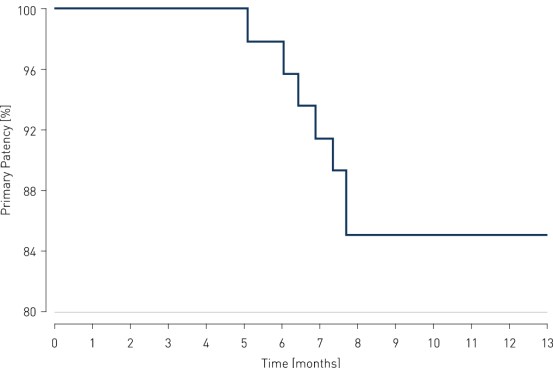
- At 12 months, results of all 36 patients show primary patency (PP) rates of 85.4% and clinically-driven freedom from Target Lesion Revascularization (FTLR) rates of 87.5%.
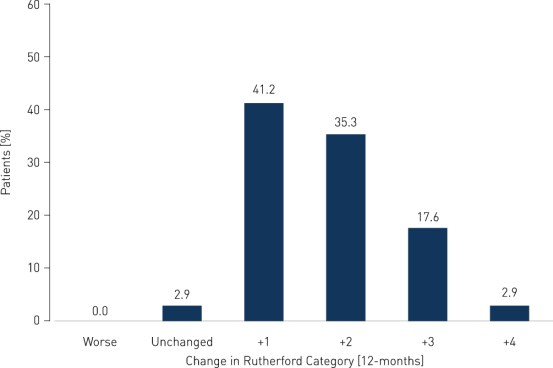
- Clinical benefit and improvement of patients' quality of life measured by improvement in Rutherford Class of 1 or more in 97.1% of patients at 12 months; ankle-brachial index (ABI) improved from 0.60 ± 0.10 before the intervention to 0.88 ± 0.08 at 12 months; and pain-free walking distance improved from 56.1 ± 34.9 m before the intervention to 654.2 ± 419.1 m at 12 months (p < 0.0001).
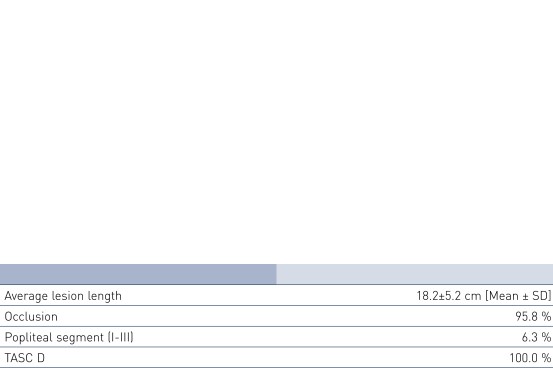
- This all-comers registry for long femoropopliteal lesions of a mean lesion length of 18.2 cm proved the Pulsar-18 stent's safe usage. Diabetes and renal insufficiency had no negative impact on rates of PP or TLR.
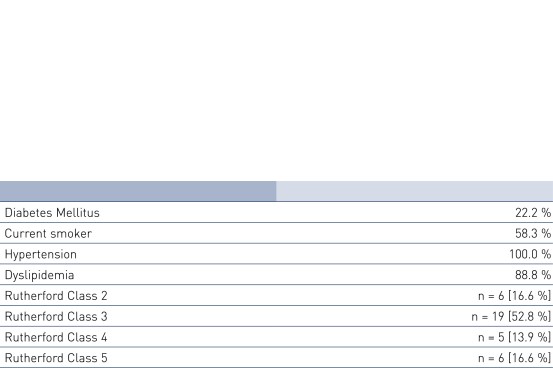
Patient Demographics and Lesion Characteristics
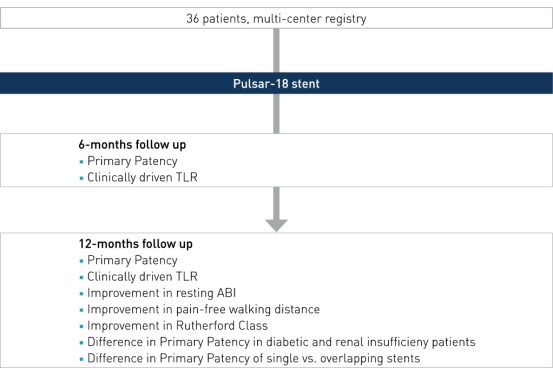
Study Design
Prospective, multi-center, investigator-initiated registry to evaluate the outcome of the implantation of the Pulsar-18 stent in lesions >15 cm in the femoropopliteal arteries.
- Number of patients (n): 36
- Principal investigator: Dr. Michael Lichtenberg, Klinikum Arnsberg, Germany
- Primary endpoint: PP at 6 and 12 months, defined as a binary restenosis on duplex ultrasound (PSVR < 2.5) at the stented target lesion with no clinically-driven TLR (cd-TLR) within the stented segment
- Secondary clinical endpoints (selected): improvement in resting ABI, improvement in pain-free walking distance, improvement in Rutherford Class, difference in PP in diabetic and renal insufficieny patients, difference in PP of single vs. overlapping stents
12-Month Results
Primary Patency and Clinical Improvement in Rutherford Class
Downloads

Vascular Intervention
Self-expanding StentOne-handed stent release for accurate stent deployment
Vascular Intervention
Self-expanding StentTri-axial shaft for a stable delivery system during stent deployment
Source:
Lichtenberg M et al. Evaluation of the 4-French Pulsar-18 Self-expanding Nitinol Stent in Long Femoropopliteal Lesions. Clinical Medicine Insights: Cardiology. 2014; 8(S2): 37–42.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
