Prospera™ Spinal Cord Stimulation System with Embrace One™
The Prospera Spinal Cord Stimulation (SCS) System with Embrace One is enhancing the SCS experience with a first-of-its-kind approach: one that’s not just implanted, but truly connected to the needs of patients and providers. Through automatic, objective, daily remote monitoring of the device and therapy, we put true proactive care* into action so that the therapy you prescribe remains optimized for each patient, every day, over the lifetime of the therapy.
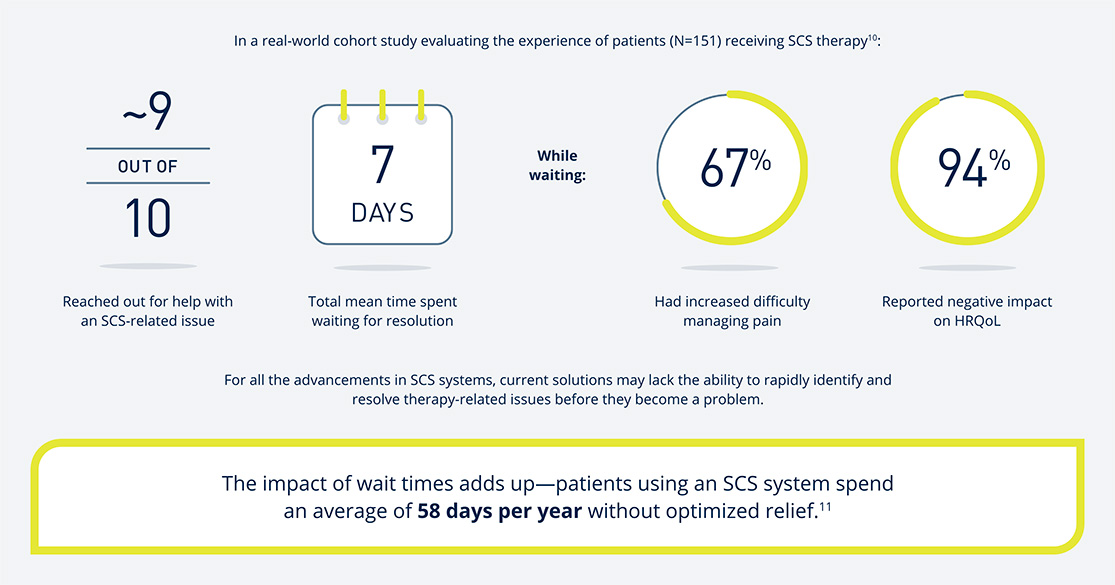
Keeping therapy optimized with conventional SCS systems can be a challenge
While clinical studies have demonstrated long-term effectiveness of SCS1-5, in the real world, loss of efficacy and explantation are commonly reported despite the introduction of advanced SCS technology6-9. Simple device issues often go undetected for far too long, which can negatively impact patients in a number of ways.
The Prospera SCS System with Embrace One offers relief like no other
PROSPERA SPINAL CORD STIMULATION WITH EMBRACE ONE
Bibi's story
Prospera SCS System
MRI for Real Life
RESONANCE™ Technology
Embrace One
BioVantage™
Clinical Evidence
Delivering an optimal SCS experience
The first and only SCS system that features:
- RESONANCE, the only multiphase stimulation paradigm available
- Embrace One, a truly first-of-its-kind, proactive care* model made possible only through automatic, objective, remote daily monitoring




All images are rendered.
MRI for real life: More access with fewer restrictions
For the 84% of SCS patients who will need at least 1 MRI within 5 years of implant,12 BIOTRONIK Neuro has them covered.
![]()
First FDA-approved SCS system that allows 1.5T and 3T full body scanning in normal operating mode with no exclusion zones
![]()
A network of MRI partners across 500+ facility locations11
![]()
Pre- and post-MRI device checks that can be conducted remotely
![]()
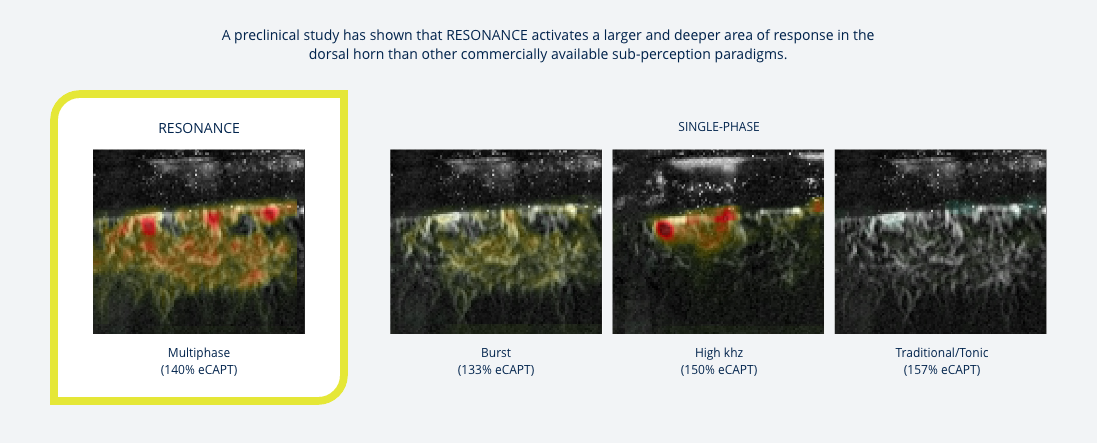
An evolution in therapeutic stimulation
RESONANCE powered by BioARCTM uses a proprietary pattern of stimulation to activate the dorsal horn where pain is processed.
- Delivers a sequence of micropulses with each unit of stimulation‡
- Stimulates and charge balances simultaneously by rapidly rotating a cathode between up to 4 selected electrodes.
- Achieves up to a 300% increase in pulse density, while reducing the charge of each micropulse.11,13§ This design is intended to eliminate the problem of overstimulation
Watch the video to see how RESONANCE multiphase stimulation works.
Shown to have a substantial effect on dorsal horn structures14§
RESONANCE drives a deeper response into the dorsal horn where pain is processed, targeting the body’s own inhibitory pathway and the natural properties of spatial and temporal summative response.
BioARC keeps programming simple and stimulation personalized
The unique stimulation pattern of BioARC allows you to focus your expertise on patient care rather than on programming.
Embrace One: Connected to what counts
The only proactive care* ecosystem that’s truly connected to the needs of patients and providers, before implant and beyond.
PATIENT BENEFITS
![]()
The only SCS system with automatic, objective, daily remote monitoring powered by HomeStreamTM to enable wireless data transmission and true proactive care
![]()
Personalized SCS therapy support 7 days a week from a dedicated Embrace One Care Team member
![]()
Embrace One App offers 24/7 access to exclusive resources and goal-tracking features
PRACTICE BENEFITS
![]()
24/7 transparency of SCS status and patient journey
![]()
Efficiently submit and track patient authorizations and psychological evaluations
![]()
Highly secure data transmission meets advanced cybersecurity standards

Daily remote monitoring and proactive care are designed to address key unmet needs
- Enabling early identification of potential issues
- Reducing time to intervention
- Minimizing time spent with nonoptimized stimulation
- Helping maintain relief and belief in therapy
The opportunity for optimization is assessed daily by the Embrace One Care Team
HomeStream transmits data from the patient’s stimulator to a secure cloud, where the data are stored for viewing by BIOTRONIK and clinicians in the Provider Portal. This automatic, objective, remote daily monitoring of 4 device-related categories enables proactive care.

For SCS therapy management and care, there is no comparison
The built-in capabilities of Embrace One go far beyond the industry standard to help support proper device usage and treatment goals.

BioVantage: Expanding support for Prospera patients
The only SCS expanded service option with built-in monthly remote patient touchpoints that complement the support and therapy optimization enabled by Embrace One.
Expanded Patient Support
Sustaining patient engagement and therapy optimization through:
![]()
Monthly patient check-ins with a skilled clinical SCS team that understands patients' specific care needs
![]()
Remote, secure sessions that allow patients to participate from the comfort of their home
![]()
In-depth discussions with patients about pain relief, therapy adherence and treatment goal progress, providing you with information needed to fine-tune treatment plans
Practice Value
Designed to meet regulatory and reimbursement guidelines for remote therapeutic monitoring,** helping clinicians make remote patient follow-up financially sustainable.
Easy Implementation
The Embrace One Care Team handles the majority of the steps in the process so you can expand your patient care without reducing clinic efficiency.

Incomparable care. Every day. Month after month.
Together, Embrace One and BioVantage go above and beyond to maintain treatment plan adherence, patient engagement, and optimal SCS therapy.

Improvement in sleep and function. Results sustained over time.15
Interim 12-month results from BENEFIT-03: a long-term efficacy and safety study (ongoing in Australia) of the Prospera SCS System with RESONANCE and automatic, daily transmission of objective device data monitoring with remote programming.15-17
As of this interim analysis not all participants had completed the 12-month follow-up. Analyses include all patients with data available at each timepoint. Outcomes may change as additional participant data are collected and monitored.
PAIN REDUCTION

IMPROVED FUNCTION

DAILY PAIN AND SLEEP IMPROVEMENTS

First-ever clinical validation of SCS and proactive care16
DAILY TRIGGERS

PROACTIVE CARE RESPONSE TIME

PATIENT AND CLINICIAN EXPERIENCE WITH REMOTE DEVICE MANAGEMENT (RDM)

OPIOID USE

Note: Embrace One is a support platform intended to help manage a patient’s experience with spinal cord stimulation. It is not intended to be used for medical diagnosis or medical treatment.
*Proactive Care: BIOTRONIK Neuro’s remote support team may reach out to patients to ensure proper usage of the spinal cord stimulator based on remotely monitored data. BIOTRONIK Neuro does not provide health advice or clinical actions outside the scope of spinal cord stimulator proper usage. This product support is not a replacement for the patient’s responsibility to communicate any medical questions or concerns with the physician’s office.
†Actual connection type varies based on location and variability.
‡Unit of stimulation=therapeutic cathodic phase along with the corresponding charge balancing anodic phase.
§As shown in pre-clinical and computational models.
¶Based on publicly available information on the commercially available SCS systems and the accompanying digital platforms offered by Abbott, Boston Scientific, Medtronic, Nevro, and Saluda as of February 2024.
#Some Abbott devices have the capabilities to perform programming adjustments over Wi-Fi and LTE.
||Features include psychologic evaluations, prior authorization services, and appointment scheduling.
**BioVantage is an elective, paid subscription offering from BIOTRONIK Neuro, Inc. All services are provided under the general supervision of a physician. Billing and coding information is subject to frequent and unexpected change; therefore, BIOTRONIK Neuro recommends that users refer to the information sources to verify accuracy prior to acting on the information provided. Submission of claims for reimbursement is solely at the discretion of the supervising physician. BIOTRONIK Neuro makes no representation or warranty regarding the accuracy, completeness, or applicability of reimbursement information, and specifically disclaims liability or responsibility for the results or consequences of any actions taken in reliance on such information.
eCAPT=evoke compound action potential threshold; EPG=external pulse generator; HRQoL=health-related quality of life; ICD=implantable cardioverter-defibrillator; IPG=internal pulse generator; MRI=magnetic resonance imaging; NRS=numerical rating scale; ODI=Oswestry Disability Index; OOR=out of range; RDM=remote device management; RF=radiofrequency; SCS=spinal cord stimulation; SQ=sleep quality; VAS=visual analogue scale.
References
1. Deer TR, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective randomized controlled trial using a novel burst waveform. Neuromod. 2018;21(1):56-66. 2. North J, Loudermilk E, Lee A, et al. Outcomes of a multicenter, prospective, crossover, randomized controlled trial evaluating subperception spinal cord stimulation at ≤1.2 kHz in previously implanted subjects. Neuromod. 2020;23:102-108. 3. Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomized, controlled trial. Lancet Neurol. 2020;19(2):123-134. 4. Fishman M, Cordner H, Justiz R, et al. Twelve-month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Practice. 2021;21:912-923. 5. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain. Anesthesiology. 2015;123(4)851-860. 6. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromod. 2015;18:603-609. 7. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromod. 2017;20(6):543-552. 8. Simopoulos T, Aner M, Sharma S, Ghosh P, Gill JS. Explantation of percutaneous spinal cord stimulator devices: a retrospective descriptive analysis of a single-center 15-year experience. Pain Medicine. 2018;0(0):1-7. 9. Hagedorn JM, Lam CM, D’Souza RS, et al. Explantation of 10 kHz spinal cord stimulation devices: a retrospective review of 744 patients followed for at least 12 months. Neuromod. 2021;24:499-506. 10. Amirdelfan K, Antony A, Levy R, et al. Patient burdens associated with spinal cord stimulation: impact of wait times to address device-related issues in a real-world cohort with chronic back and leg pain [WIP abstract P-136]. Pain Pract. 2022;22(S1):25-2. 11. BIOTRONIK Neuro data on file. 12. Desai MJ, Hargens LM, Breitenfeldt MD, et al. The rate of magnetic resonance imaging in patients with spinal cord stimulation. Spine. 2015;40(9):e531-e537. 13. Riahi P, Slee S, Kibler A, Muessig D, Stotts L. Computational model-based evaluation of novel multi-phase neuromodulation. Neuromod. 2020;23:e1-e325. 14. Amirdelfan K, Falowski S, Slee S, Kibler A. Functional ultrasound imaging reveals activation properties of clinical spinal cord stimulation therapy. Poster presented at: North American Neuromodulation Society Annual Conference; January 13, 2023; Las Vegas NV. 15. Russo M, Yu J, Mohabbati V, Amirdelfan K, Kapural L, Verrils P. Long-term study of SCS system with multiphase stimulation and remote device management: interim 12-month results. Poster presented at: North American Neuromodulation Society Annual Conference; January 18, 2024; Las Vegas, NV. 16. Russo M, Yu J, Mohabbati V, Amirdelfan K, Kapural L, Verrils P. Proactive remote device management enables rapid SCS optimization: results from a prospective multicenter study. Poster presented at: North American Neuromodulation Society Annual Conference; January 18, 2024; Las Vegas, NV. 17. Russo M, Yu J, Mohabbati V, Amirdelfan K, Kapural L, Verrils P. Remote SCS management reduces patient burdens and improves care: results from a prospective multicenter study. Poster presented at: North American Neuromodulation Society Annual Conference; January 18, 2024; Las Vegas, NV.
![]()
Brief Summary: Please reference the appropriate product Instructions for Use (IFU) for more information regarding indications, contraindications, warnings, precautions, and potential adverse events. Indications for Use: The Prospera™ Spinal Cord Stimulation (SCS) System is indicated as an aid in the management of chronic, intractable pain in the trunk and/or limbs, which may include unilateral or bilateral pain. Contraindications: Implantation of a spinal cord stimulator may be contraindicated in patients who are unable to operate the SCS system, or who have failed to receive effective pain relief during SCS trial stimulation, or who are poor candidates for surgery. Note that the safety and effectiveness of Prospera SCS system has not been established in pediatric patients or pregnant or nursing patients. Warnings: The following may cause electromagnetic interference, adverse interactions, insufficient or excessive stimulation, damage and function loss of the system, and/or therapy failure: external defibrillation, transcutaneous electrical nerve stimulation (TENs), lithotripsy, RF ablation, hyperbaric oxygen therapy, electrocautery, diathermy therapy (including shortwave, microwave, and therapeutic ultrasound therapies), high-power ultrasound, radiation therapy, Magnetic Resonance Imaging (MRI) scan (refer to Prospera SCS System MRI Guidelines for the system’s MR conditional information), use of portable RF communication equipment near the SCS system, use of a non-BIOTRONIK-provided charger. The Prospera SCS System may interfere with the operation of implanted pacemakers or ICDs. The effects of an implanted Prospera SCS System on other neurostimulators are unknown. Precautions: Device malfunction, loss of therapy, and other adverse events including patient injury may occur if the device is not handled or operated properly as described in the IFU. Refer to the product IFU for comprehensive safety messages when handling the device. Potential Adverse Events: Risks associated with SCS system placement: pain at the implant site, infection, cerebrospinal fluid (CSF) leakage, CSF fistula, epidural hemorrhage, bacterial meningitis, seroma, hematoma, paralysis. Additional risks associated with SCS system use: lead migration; stimulator migration; allergic response or tissue reaction to the implanted system material; skin erosion; radicular chest wall stimulation; disturbed urination; dysesthesia; decubitus; premature battery depletion; and uncomfortable stimulation or ineffective pain management. Furthermore, there is the risk that the SCS therapy may not be effective in relieving symptoms or may cause worsening of symptoms. Refer to the product IFU for a comprehensive list of potential adverse events.

