Orsiro® Mission DES

Thinnest Drug-Eluting Stent System Offering Enhanced Deliverability.
Find out how Orsiro® Mission can benefit your daily practice
Orsiro® Mission is a new drug-eluting stent system that offers advanced deliverability and an ultrathin strut design considered the thinnest available in the United States. It features the same proprietary design and innovative bioabsorbable coating with controlled drug release as the Orsiro stent. Improvements to the new Orsiro Mission DES system include a re-engineered delivery system and a new deep embedding process to improve deliverability further, including ‘best in class’ trackability and crossability2.
 Next level of deliverability1
Next level of deliverability1
 Ultrathin struts2
Ultrathin struts2
 Outstanding patient outcomes3
Outstanding patient outcomes3
To learn more, visit the Orsiro Mission website.
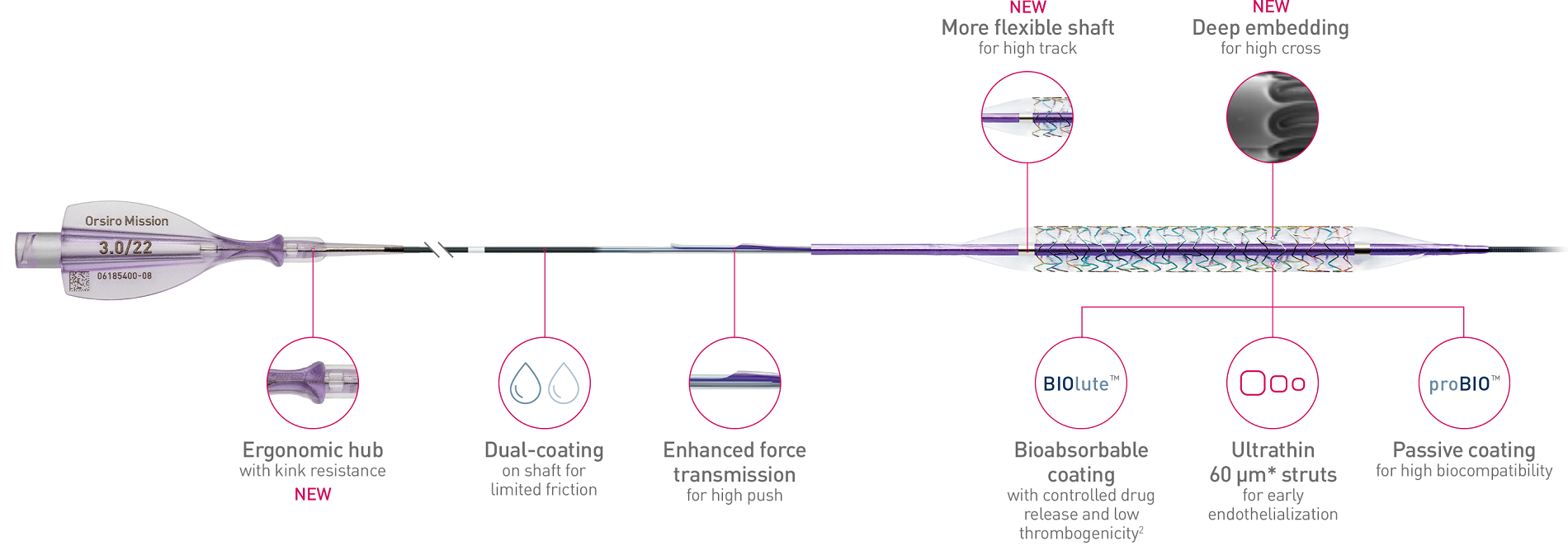
Better push, track and cross
The next level of deliverability
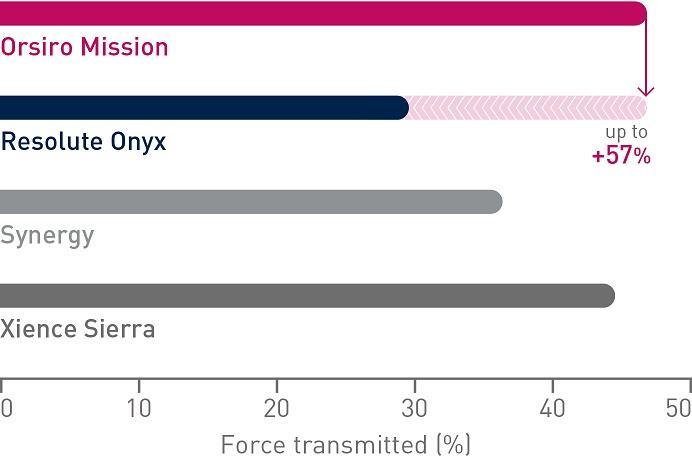
1st in Push4
Transmits up to 57% more force from hub to tip4
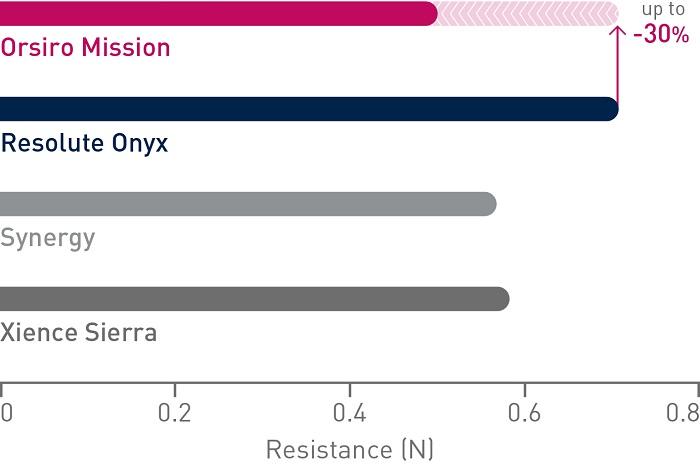
1st in Track4
Up to 30% less force needed to follow the path
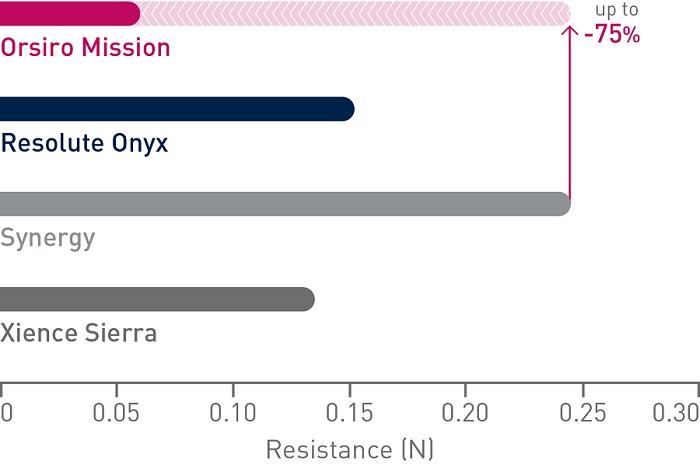
1st in Cross4
Up to 75% less force needed to successfully cross demanding anatomies
Explore our extensive clinical program
Outstanding patient outcomes³
BIOFLOW-V Trial. TLF and components at 12, 24, and 36 Months
- 40% lower TLF rate20ф (p = 0.003)
- 46% lower TV-MI rate20ф (p = 0.004)
- 52% lower Ischemia-driven TLR rate20ф (p = 0.008)
¤ p-values for 36-m frequentist analysis of BIOFLOW-V.20ф // ф vs. Xience, based on 36-m frequentist analysis of BIOFLOW-V.20
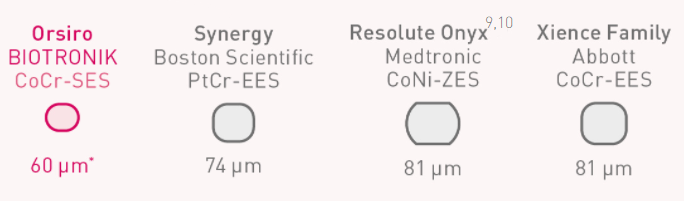
Discover why thinner struts make the difference
Ultrathin Struts
Orsiro Mission DES is an ultrathin strut2 that is considered the thinnest available in the U.S.6
• Less disrupted flow
• Improved re-endothelialization
* Nominal strut thickness for size ø 2.25 - 3.0 mm, mean diameter 62 μm.
Technical Data
Indication: The Orsiro® Mission Sirolimus-Eluting Coronary Stent System is a drug-eluting balloon-expandable stent pre-mounted on a fast-exchange PTCA catheter delivery system. It is indicated for improving coronary luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease, stable angina, unstable angina, non-ST-elevation myocardial infarction or documented silent ischemia due to atherosclerotic lesions in the native coronary arteries with a reference vessel diameter of 2.25 mm to 4.0 mm and a lesion length of ≤ 36 mm.
Technical Data Stent
Stent material Cobalt chromium, L-605
Passive coating proBIO™ amorphous silicon carbide
Active coating BIOlute™ bioabsorbable drug matrix consisting of
sirolimus and polymer poly-l-lactide (PLLA)
Nominal drug content 1.4 μg/mm²
Delivery system
Catheter type Fast-exchange
Recommended guide catheter 5 F (min. I.D.Ж ≥ 0.056“)
Guide wire diameter 0.014” (0.36 mm)
Usable catheter length 140 cm
Balloon material Semi crystalline polymer
Coating (distal shaft) Hydrophilic
Coating (proximal shaft) Hydrophobic
Marker bands Two swaged platinum-iridium markers
Proximal shaft diameter 2.0 F
Distal shaft diameter 2.7 F: ø 2.25 – 3.0 mm; 2.9 F: ø 3.5 – 4.0 mm
Nominal pressure (NP) 10 atm
Rated burst pressure (RBP) 16 atm
