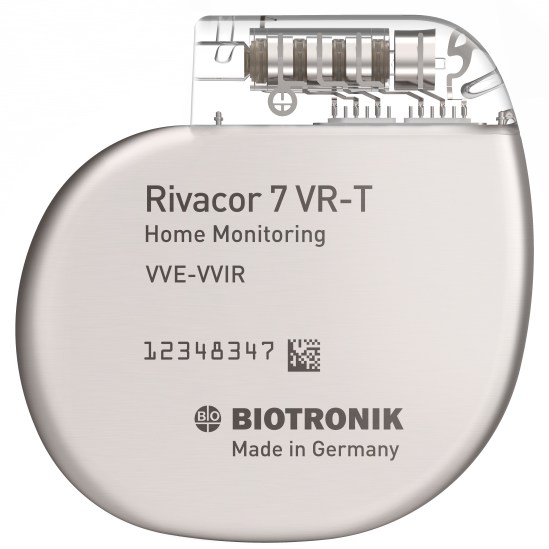



Rivacor 7 VR-T
MR CONDITIONAL SINGLE-CHAMBER ICD
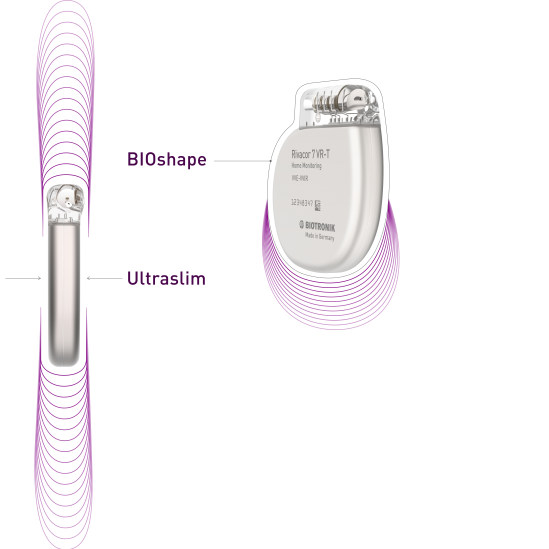
Rivacor devices incorporate more diagnostic and therapeutic capabilities into smaller devices. The Ultraslim BIOshape eases implantation1, features a thinner form factor and a more rounded, elliptical design for reduced skin pressure. Rivacor devices are the smallest and slimmest ICDs approved for 3T full-body MRI scans2. Rivacor ICDs have an extended battery life of nearly 15 years3, supported by extended warranties, to deliver the best patient care.
The Rivacor ICD series offers ProMRI, MRI AutoDetect, Closed Loop Stimulation (CLS), BIOTRONIK Home Monitoring®, Extended Longevity, and MorphMatch.
KEY FACTS
- ProMRI4: 3T full-body scan MRI access
- MRI AutoDetect: Minimize time in MRI mode, increase provider efficiency, expand scheduling flexibility, reduce administrative burden
- Closed Loop Stimulation (CLS): Only BIOTRONIK ICDs offer physiologic rate adaptation; this algorithm responds to physical exertion and acute mental stress
- BIOTRONIK Home Monitoring® with QuickCheck and IN-TIME template: Most user-friendly system with highest patient compliance and daily transmission, up to 38% reduction in mortality as demonstrated by the TRUECOIN study5
- Extended Longevity: Estimates include CLS and daily Home Monitoring transmissions
- MorphMatch: SVT discrimination based on QRS morphology analysis provides insight into discrimination decision
1 Post-market observation; interim-analysis, December 21, 2018. Data on file.
2 As part of an MR conditional system.
3 Remote Monitoring: Daily Transmissions, 3 Channel IEGM: On, Shelf Life: 6months, Semi-annual shocks, 60bpm, RV: 700Ω, 2V@04ms, 15% pacing.
4 For combination of MR conditional devices, please see the "ProMRI MR conditional device systems" manual.
5 Hindricks G et al. Eur Heart J. 2017, 38(22).


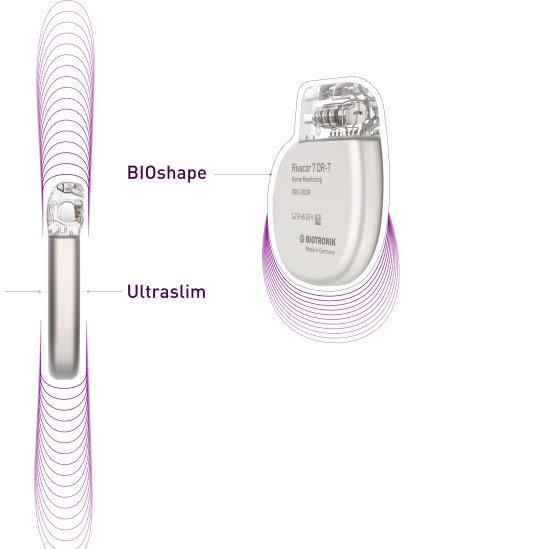



Rivacor DR-T
MR CONDITIONAL DUAL-CHAMBER ICD
Rivacor devices incorporate more diagnostic and therapeutic capabilities into smaller devices. The Ultraslim BIOshape eases implantation1, features a thinner form factor and a more rounded, elliptical design for reduced skin pressure. They are the smallest and slimmest ICDs approved for 3T full-body MRI scans2. Rivacor ICDs have an extended battery life of nearly 15 years3, supported by extended warranties, to deliver the best patient care.
The Rivacor DR-T ICD series offers ProMRI, MRI AutoDetect, Closed Loop Stimulation (CLS), BIOTRONIK Home Monitoring®, Extended Longevity, and Automatic Atrial ATP.
KEY FACTS
- ProMRI4: 3T full-body scan MRI access
- MRI AutoDetect: Minimize time in MRI mode, increase provider efficiency, expand scheduling flexibility, reduce administrative burden
- Closed Loop Stimulation (CLS): Only BIOTRONIK ICDs offer physiologic rate adaptation; this algorithm responds to physical exertion and acute mental stress
- BIOTRONIK Home Monitoring® with QuickCheck and IN-TIME template: Most user-friendly system with highest patient compliance and daily transmission, up to 38% reduction in mortality as demonstrated by the TRUECOIN study5
- Extended Longevity: Estimates include CLS and daily Home Monitoring transmissions turned ON
- Automatic Atrial ATP: Delivers atrial therapies to automatically treat AT/AF episodes
1 Post-market observation; interim-analysis, December 21, 2018. Data on file.
2 As part of an MR conditional system.
3 Remote Monitoring: Daily Transmissions, 3 Channel IEGM: On, Shelf Life: 6months, Semi-annual shocks, 60bpm, RV: 700Ω, 2V@04ms, 15% pacing.
4 For combination of MR conditional devices, please see the "ProMRI MR conditional device systems" manual.
5 Hindricks G et al. Eur Heart J. 2017, 38(22).
