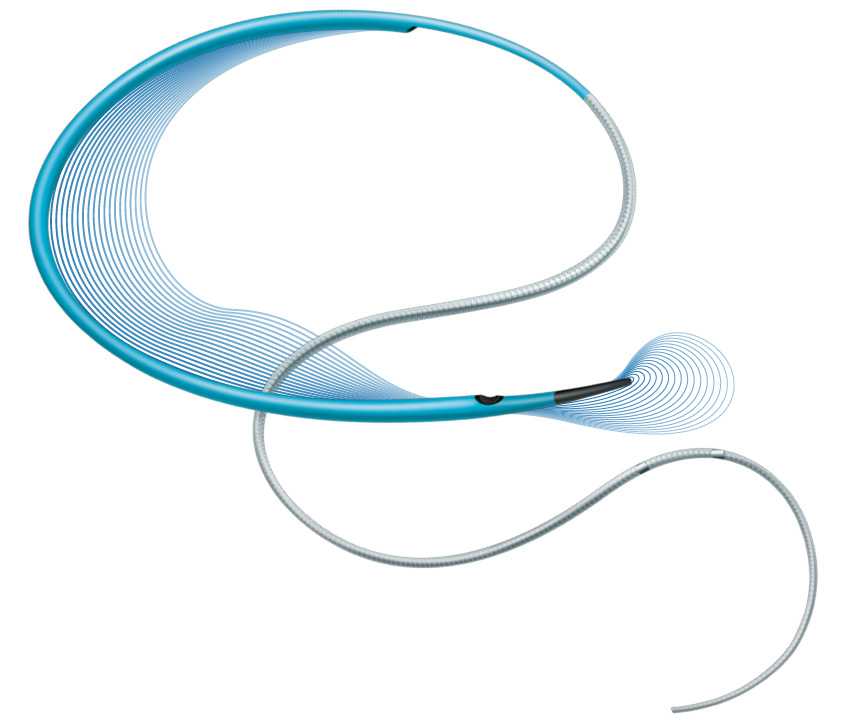
Dual Lumen Microcatheter
NHancer Rx
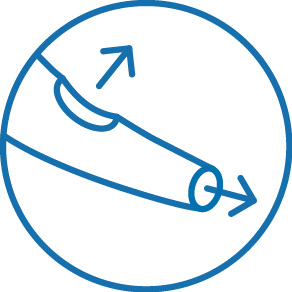
Two distal exit ports for enhanced access of side branches
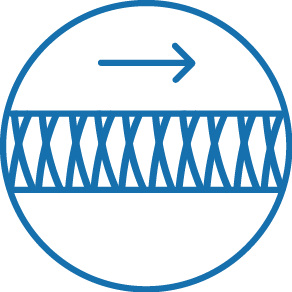
Braid reinforced shaft for improved pushability
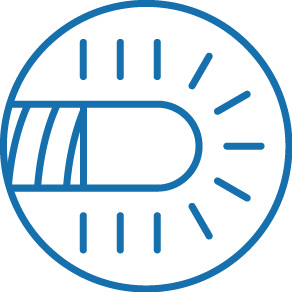
Soft, tapered tip with distal end visibility
NHancer Rx
Dual Lumen Microcatheter
Indicated to support a guide wire during access of vasculature.*
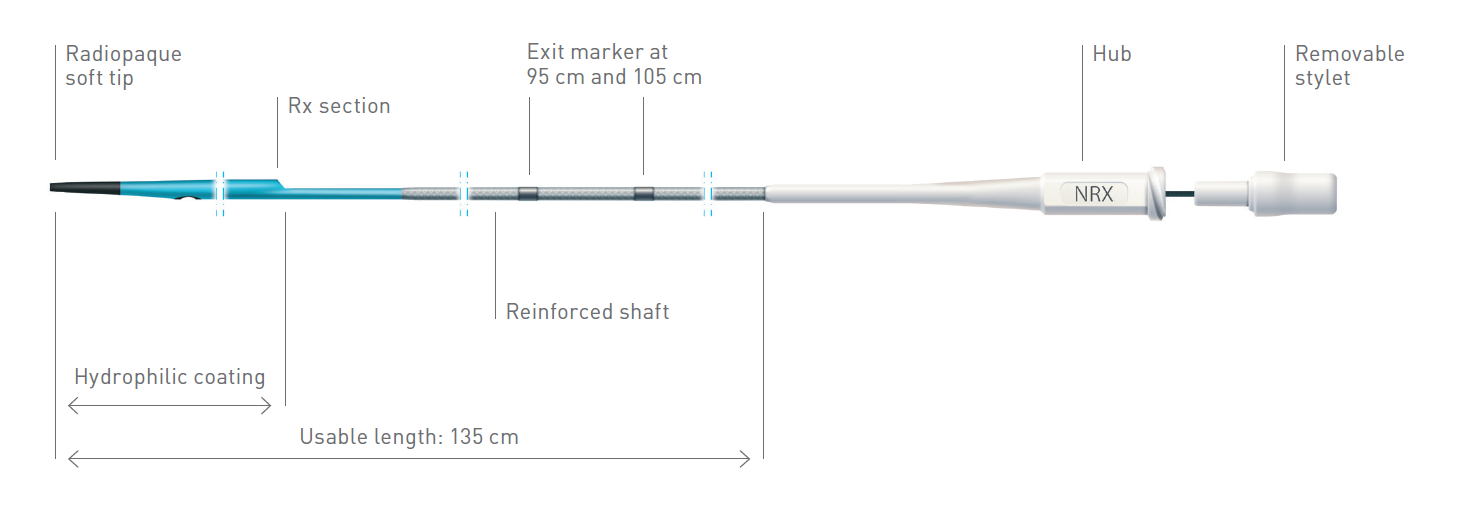
The NHancer Rx dual lumen microcatheter has one over the wire (OTW) lumen and one rapid exchange (Rx) delivery lumen. The two separate lumens are designed to advance dual guide wire procedures by providing two distal exit ports for enhanced access of side branches. The reinforced shaft and removable stylet facilitates pushability and the soft, tapered tip is designed to be atraumatic.
Technical Data
| Dual lumen microcatheter | |
|---|---|
| Distal profile | Oval low profile design 2.3F x 3.3F |
| Lesion entry profile | 1.5F |
| Guide wire compatibility OTW lumen | 0.014" |
| Guide wire compatibility Rx lumen | 0.014" |
| Distal shaft coating | Hydrophilic |
| Exit markers | 95 cm and105 cm |
| Tip design | Soft tapered tip |
| Ordering Information | Order Number | Model | Guiding catheter compatibility | Usable length | ||||||||||
| 451669 | NRX1413518 | 5F | 135 cm | |||||||||||

Contact
*Indication as per IFU
NHancer Rx is a trademark of IMDS.
Distributed by BIOTRONIK in selected countries.
Manufacturer:
IMDS Operations B.V.
Ceintuurbaan Noord 150
9301 NZ Roden,
The Netherlands
Tel +31 (0)50 8200230
info@imds.nl


