Drug-Eluting Stent System
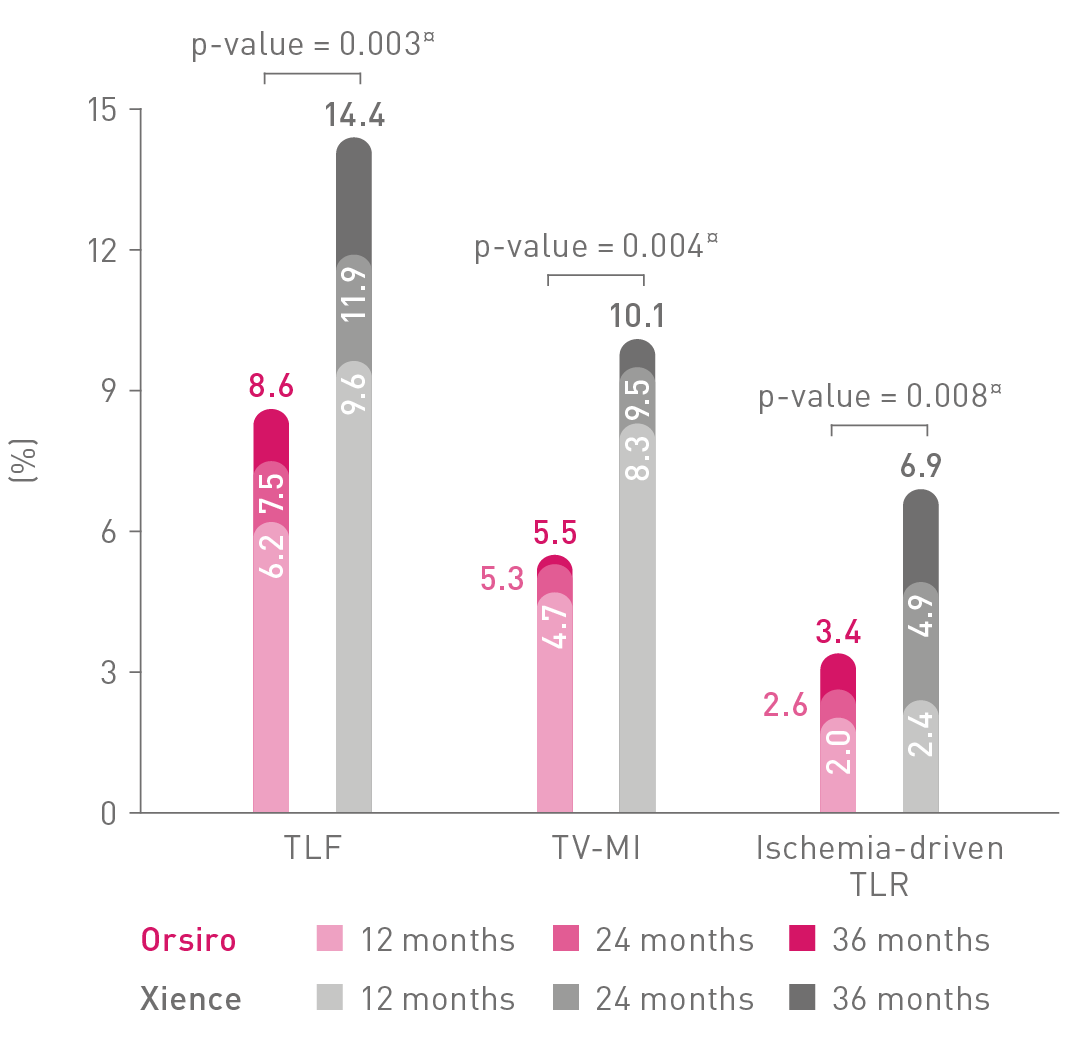
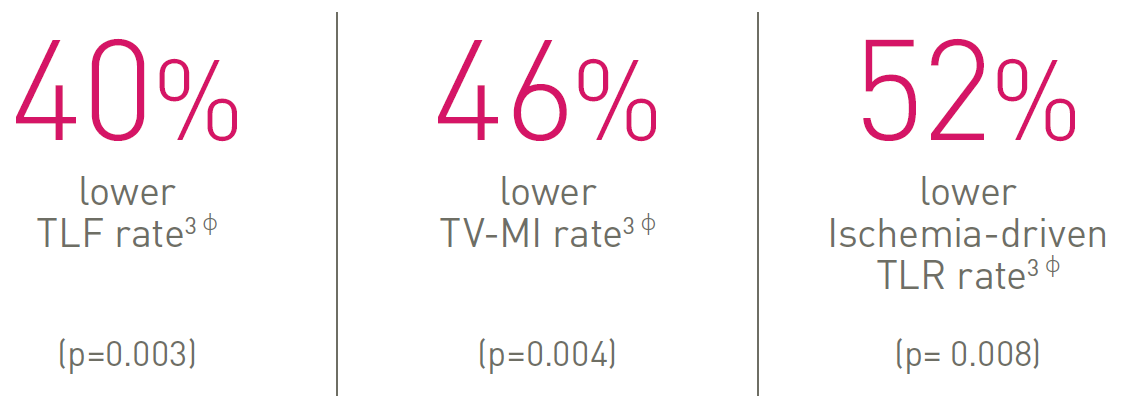
TLF – Target Lesion Failure; TV-MI – Target Vessel Myocardial Infarction; TLR – Target Lesion Revascularization.
§As characterized with respect to strut thickness in Bangalore et al. Meta-analysis.9
◊Based on investigator’s interpretation of BIOFLOW-V primary endpoint results.
*Compared to Xience, based on three consecutive years.
¤p-values for 36-m frequentist analysis.
ф vs. Xience, based on 36-m frequentist analysis.

thinnest available in the US5



‡ Driven by peri-procedural MI events (<48 hours). In-hospital rate may include events > 48 hours.
Δ Images: Secco G et al. Time-related changes in neointimal tissue coverage following a new generation SES implantation: an OCT observational study. Presented at: euro PCR, May 20, 2014; Paris, France.


ф vs. Xience, based on 36-m frequentist analysis.




Dr. Dean Kereiakes
BIOFLOW-V Site Principal Investigator
Technical Data
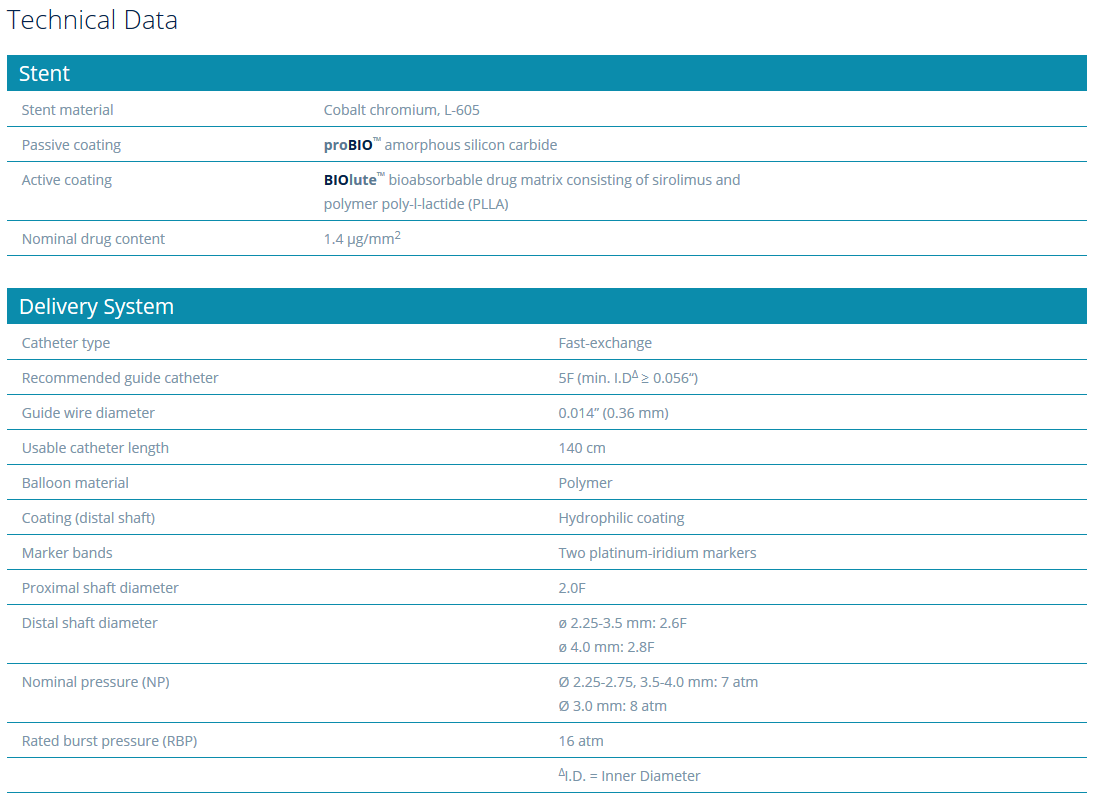
Compliance Chart
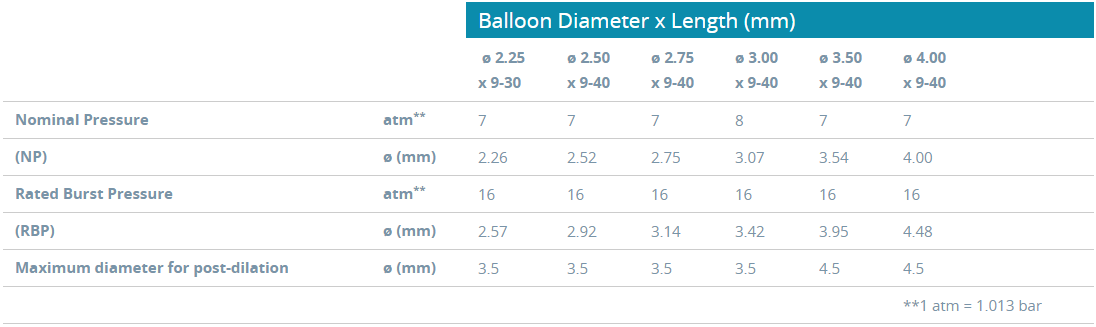
Ordering Information
Contact
1. Kandzari D et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimuseluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. October, 2017; 2. Kandzari D et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus- Eluting Stents: Journal of American College of Cardiology. 2018, doi: https//doi.org/10.1016/j.jacc.2018.09.019; 3. Kandzari D et al. J Am Coll Cardiol. Cardiovasc Interven. 2020, doi: 10.1016/j.jcin.2020.02.019; 4. Pilgrim T et al. 5-year outcomes of the BIOSCIENCE randomised trial. Supplementary appendix. Lancet, August, 2018; 5. When compared to FDA approved Drug Eluting Stents. BIOTRONIK data on file; 6. Foin N et al. Int J of Cardiol. 2014, 177(3); 7. Secco G et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine 17.1 (2016): 38-43; 8. Buiten R et al. Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels - A prespecified analysis of the randomized BIO-RESORT trial; JAMA Cardiol. Published online May 21, 2019. doi:10.1001/jamacardio.2019.1776; Clinical Trials. gov: NCT01674803; 9. Bangalore S et al. Circulation. 2018, 138; 10. BIOTRONIK data on file; IIB(P)24/2018.
Synergy is a trademark or registered trademark of the Boston Scientific Group of Companies; Resolute, Integrity, Resolute Integrity and Resolute Onyx are trademarks or registered trademarks of the Medtronic Group of Companies; Xience, Xience Prime and Xience Xpedition are trademarks or registered trademarks of the Abbott Group of Companies.
Orsiro, proBIO and BIOlute are trademarks or registered trademarks of the BIOTRONIK Group of Companies.




