TRUST
Lumos-T Safely Reduces Routine Office Device Follow-up
Demonstrate that the use of the BIOTRONIK Home Monitoring system can safely reduce the number of regularly scheduled office follow-up visits compared to the conventional method of ICD follow-up.
Study Design
- Prospective, randomized, multicenter
- 1,339 subjects randomized 2:1 to HM or conventional follow-up at 102 centers
Study Design
Home Monitoring delivered a reduction of 45% of in-office follow-ups1
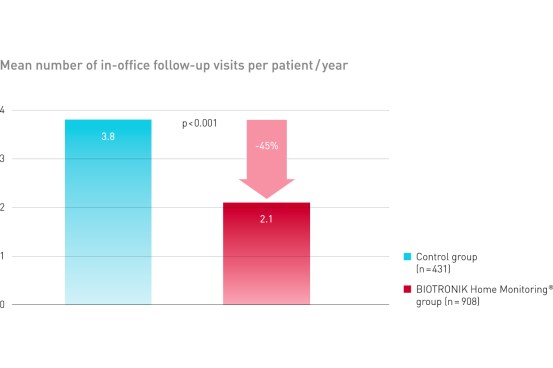
Clinical Relevance
BIOTRONIK Home Monitoring had the same safety event rate as the control group1
Early Detection
Home Monitoring detected arrhythmias 27-37 days (median) in advance of conventional office follow-up1
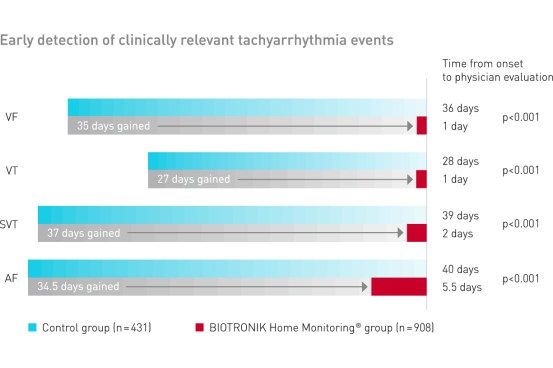
Clinical Relevance
- Home Monitoring safely reduced device follow-up burden
- Home Monitoring provides early detection of clinically relevant events
- Home Monitoring improves the efficiency of device patient office visits
| Reference no. |
|
|---|---|
| Study Objective |
|
| 1° Endpoints |
|
| 2° Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Devices |
|
| Follow-Up |
|
| Study Duration |
|
| Principal Investigators |
|
Downloads
Downloads
Related Products

Tachycardia Therapy
BIOTRONIK offers an extensive product portfolio in the area of tachycardia therapy.
Clinical Relevance
1 Varma N, Epstein AE, Irimpen A, Schweikert R, Love C; TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010 Jul 27;122(4):325-32. doi: 10.1161/CIRCULATIONAHA.110.937409. Epub 2010 Jul 12.
3 Varma N, Pavri BB, Stambler B, Michalski J; TRUST Investigators. Same-day discovery of implantable cardioverter defibrillator dysfunction in the TRUST remote monitoring trial: influence of contrasting messaging systems. Europace. 2013 May;15(5):697-703. doi: 10.1093/europace/eus410. Epub 2012 Dec 19.
Clinical Relevance
2 Varma N, Michalski J, Stambler B, Pavri BB; TRUST Investigators. Superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial - testing execution of the recommendations. Eur Heart J. 2014 May 21;35(20):1345-52. doi: 10.1093/eurheartj/ehu066. Epub 2014 Mar 3.
4 Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol. 2010 Oct;3(5):428-36. doi: 10.1161/CIRCEP.110.951962. Epub 2010 Aug 17.
